Development of a Semi-Crystalline Binder with Electrical Conductivity and Underwater Adhesion by Professor Dongwook Lee's Team
Improved Lifespan and Energy Efficiency of Seawater Batteries... Published in Energy Environ. Sci.
A new material has been developed that can simultaneously improve both the lifespan and energy efficiency of seawater batteries, which store electricity using seawater.
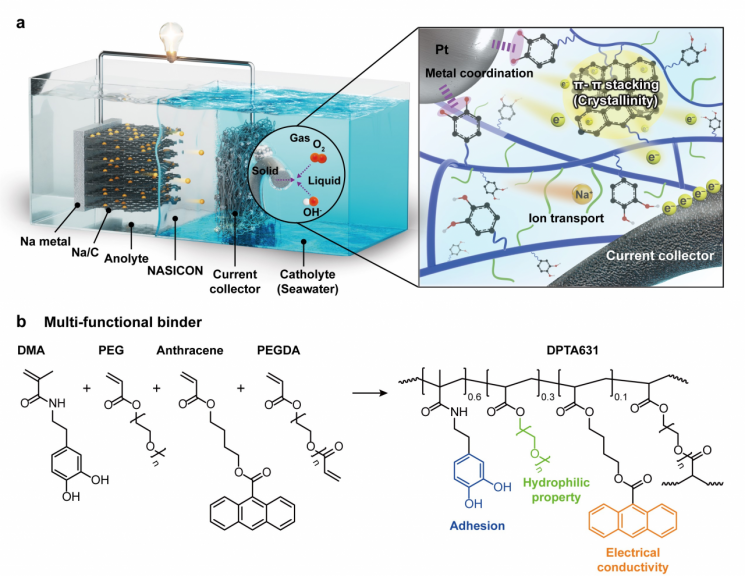
The research team led by Professor Dongwook Lee from the Department of Energy and Chemical Engineering at UNIST has developed a semi-crystalline polymer binder with excellent electrical conductivity and underwater adhesion.
 Professor Dongwook Lee. Provided by UNIST
Professor Dongwook Lee. Provided by UNIST
The battery electrode is a composite structure made of several materials, and the binder that firmly adheres these materials together determines its performance. In particular, since seawater batteries must operate for long periods underwater, it was essential to develop a binder that not only possesses underwater adhesion but also conducts electricity well.
The semi-crystalline binder designed by the research team features a structure where amorphous and crystalline regions are mixed within a single material, resulting in excellent adhesion and electrical conductivity.
The crystalline regions, where polymers are regularly arranged, provide straight pathways for electrons to move, thereby enhancing electrical conductivity. Meanwhile, in the amorphous regions, the polymer chains move flexibly and easily bond with surfaces, contributing to adhesion.
When the newly developed binder was applied, the battery's lifespan increased by 3.3 times compared to those using the conventional PVDF binder. The conventional PVDF binder showed a rapid performance decline within 120 hours of initial operation, but the new binder demonstrated mid-term stability for over 400 hours and a long-term lifespan of up to 1,200 hours.
The overvoltage, which affects reaction efficiency, was also reduced by up to 66%, enabling the battery to operate with less energy under the same conditions. Additionally, the amount of electrical energy that could be extracted during discharge compared to charging increased by 26%, and the maximum output rose by 96%.
The research team identified the reasons for these performance improvements through analyses of the binder's crystallinity, electrical conductivity, and adhesion. The analysis revealed that the crystalline regions not only provided straight, high-speed pathways for electron movement, but also created randomly oriented, multi-directional electron flow routes. Furthermore, the excellent adhesion was attributed to metal coordination bonds formed between the binder and metal catalyst particles in the amorphous regions.
The newly developed binder does not contain fluorinated compounds, giving it potential as a battery material for electric vehicles that complies with the European Union's PFAS regulations. The European Union is currently pursuing a phased ban on the use of perfluorinated compounds, including PVDF, due to concerns about environmental persistence and human health risks.
Jungwook Hwang, the first author and researcher, stated, "This study demonstrates that the limitations of conventional binders can be overcome and the performance of seawater batteries can be improved through the design of semi-crystalline polymers," adding, "We expect this approach to be expanded to a variety of electronic materials, aqueous metal batteries, and energy storage systems in the future."
 Researcher Jungwook Hwang. Provided by UNIST
Researcher Jungwook Hwang. Provided by UNIST
An aqueous metal battery is a type of battery that uses a water-based electrolyte and employs metals such as lithium or sodium as electrodes. Seawater batteries are a form of aqueous metal battery that use seawater as the electrolyte.
This research was conducted in collaboration with Professors Seokju Kang, Hyunwook Lee, Youngsik Kim, and Hyunhyup Ko from the Department of Energy and Chemical Engineering at UNIST, as well as Professor Taejoo Shin from the Graduate School of Semiconductor Materials and Components. It was supported by the National Research Foundation of Korea's Mid-Career Researcher Support Program, Creative Challenge Research Support Program, and Nano and Materials Technology Development Program.
The research results were published on March 31 in Energy & Environmental Science (IF 32.4), the most prestigious academic journal in the energy field.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.