KDCA Launches Full-Scale "National Rare Disease Registration Project"
Establishing Tailored Policies Based on Disease-Specific National Statistics
The Korea Disease Control and Prevention Agency (KDCA) announced on April 23 that it will officially launch the "National Rare Disease Registration Project" to continuously and systematically collect, analyze, and support data on the occurrence, diagnosis, and treatment status of 1,314 nationally managed rare diseases.
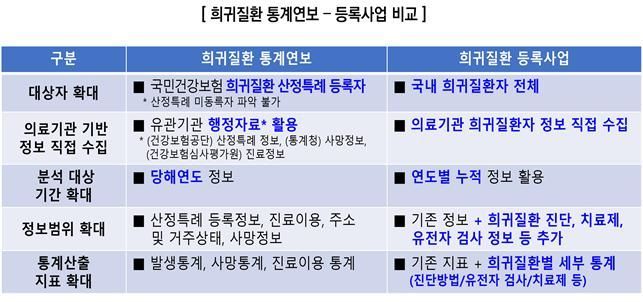
Since 2020, the "Annual Statistical Report on Rare Disease Patients" has been published and made public each year. However, the existing statistical reports have been limited to information such as the number of registered patients under the health insurance special case system, deaths, and medical service utilization for the given year, making it difficult to fully grasp the overall scale of rare disease patients and the status of each disease.
With the full-scale implementation of this medical institution-based rare disease registration project, it will become possible to directly collect patient information, disease, diagnosis and treatment information, and genetic testing data at medical sites. This will enable the identification of various disease-specific statuses that are difficult to ascertain using only the special case system information.
The information collected from medical institutions will be verified and analyzed, then used as foundational data for statistical production. It is expected to help establish data-driven, tailored policies for rare disease patients and improve early diagnosis and treatment outcomes.
Previously, to ensure the smooth implementation of the project, the KDCA designated the Korea Health Information Service as the Rare Disease Registration Project Headquarters prior to the project's launch and developed a registration management system. In addition, to collect disease-specific treatment information, the KDCA collaborated with the Ministry of Food and Drug Safety to link the system with information on rare drug designation and pharmaceutical approvals.
Furthermore, to ensure seamless project operation, a pilot operation of the registration project was conducted starting in October last year. A total of 2,824 rare disease cases were collected from 17 medical institutions, including regional rare disease specialist centers. Feedback was gathered on operational challenges and areas for improvement, and the project implementation system was pre-examined.
Ji Youngmi, Commissioner of the KDCA, stated, "Through the rare disease registration project, a new system has been established for the government to directly identify and manage the scale and status of rare diseases in Korea. Going forward, we will expand the number of participating medical institutions to achieve full registration of domestic rare disease patients. Through this, we will strive to produce more accurate national statistics and use them to develop tailored policies."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.