Interim RWD Results for Dyslipidemia Patients Presented in Poster Session
 Professor Kim Hye-kyung of the Department of Endocrinology at Severance Hospital explained the efficacy and safety of switching to 'Livalojet' at the symposium of the Korean Society of Lipid and Atherosclerosis Spring Conference held on the 5th. Photo by JW Choongwae Pharmaceutical
Professor Kim Hye-kyung of the Department of Endocrinology at Severance Hospital explained the efficacy and safety of switching to 'Livalojet' at the symposium of the Korean Society of Lipid and Atherosclerosis Spring Conference held on the 5th. Photo by JW Choongwae Pharmaceutical
JW Pharmaceutical announced on the 14th that it confirmed the LDL-cholesterol level improvement effect in patients who switched from statin monotherapy to 'Rivarozet' for the treatment of dyslipidemia.
Rivarozet is a dual-combination drug that combines 'pitavastatin' and 'ezetimibe,' ingredients used to treat dyslipidemia. It is the first domestically developed improved new drug combining pitavastatin and ezetimibe among statin formulations in Korea.
The research team led by Professor Kim Sang-hyun at the Cardiovascular Center of Boramae Medical Center in Seoul is analyzing the efficacy and safety over 48 weeks after switching to 'Rivarozet' in 7,197 patients with dyslipidemia. The team disclosed the study results for 2,221 patients initially enrolled in the clinical phase in poster form at the Korean Society of Lipid and Atherosclerosis Spring Conference held on the 5th.
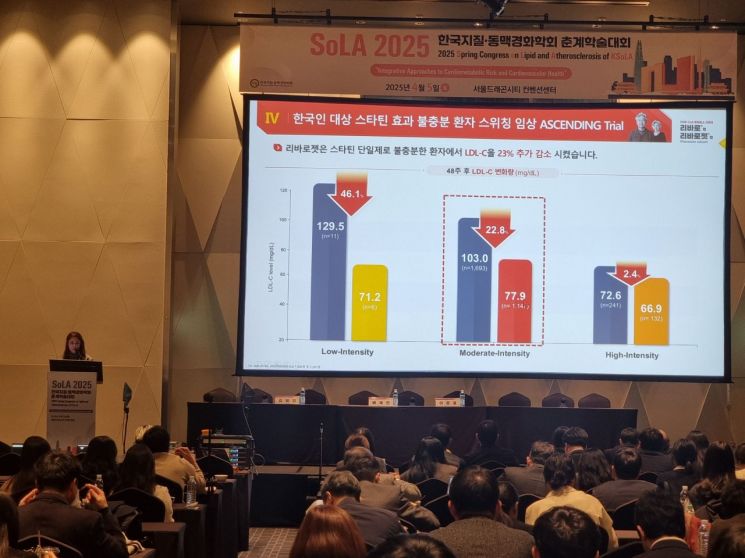
The study results showed that LDL-cholesterol levels steadily decreased at 8, 24, and 48 weeks after switching from six statin monotherapies, including atorvastatin and rosuvastatin, to 'Rivarozet.' The LDL-cholesterol level, which was 99.41 mg/dL during statin monotherapy, decreased by 22.9% to 76.69 mg/dL 48 weeks after switching to 'Rivarozet.'
In particular, LDL-cholesterol levels in patients taking moderate-intensity statin monotherapy improved from 102.98 mg/dL to 77.85 mg/dL after switching, a 24.4% improvement.
Among dyslipidemia patients with diabetes who were taking moderate-intensity statin monotherapy, LDL-cholesterol levels also significantly decreased. Eight weeks after switching to 'Rivarozet,' LDL-cholesterol levels in these patients dropped by 19.87 mg/dL and maintained a similar level thereafter.
The research team explained, "Switching patients who have insufficient effects with existing statin monotherapy to a combination of pitavastatin and ezetimibe can be an effective treatment option that induces additional LDL-cholesterol reduction."
Meanwhile, JW Pharmaceutical held a symposium on this research topic at the conference. Professor Kim Hye-kyung of the Endocrinology Department at Severance Hospital, who gave the presentation, explained at the symposium, "In terms of safety, the patients' glycated hemoglobin (HbA1c) levels were 6.58% at baseline and 6.52% 48 weeks after switching, and fasting blood glucose levels slightly decreased from 117.57 mg/dL to 115.65 mg/dL."
JW Pharmaceutical plans to continuously expand its market position by proving the therapeutic value of 'Rivarozet' for patients based on various clinical data.
A JW Pharmaceutical official stated, "We will continue to accumulate evidence-based data to enhance treatment satisfaction for healthcare professionals and patients in the future," adding, "Based on this, we will accelerate the growth of 'Rivarozet' as an original specialty drug in the dyslipidemia treatment market."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















