First Domestic Emotional Disorder Digital Therapeutic Device
Clinical Effectiveness Confirmed for Patients with Severe Anxiety Disorder
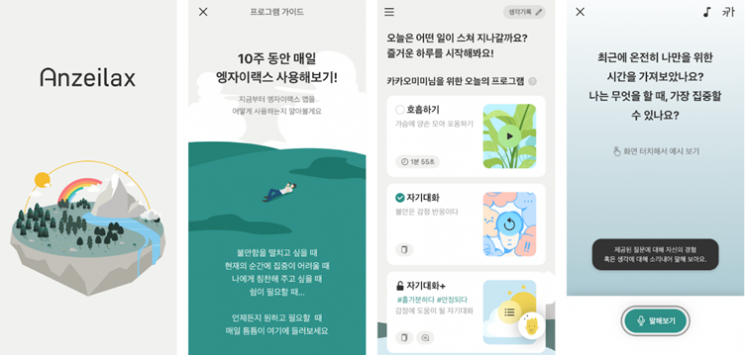
Digital therapeutics (DTx) specialist company Hi announced on the 9th that its generalized anxiety disorder (GAD) digital therapeutic device 'Anzeilax' has obtained product approval from the Ministry of Food and Drug Safety.
Anzeilax is a software medical device that provides self-talk training based on Acceptance & Commitment Therapy (ACT) to improve generalized anxiety disorder. Through processes such as cognitive defusion, mindfulness, and acceptance, it emphasizes experiencing and accepting one’s thoughts, feelings, and sensations as they are. Its core function is to provide effective self-focused shifts and balanced 'self-talk.'
Hi confirmed through clinical trials that the experimental group using the Anzeilax product for 10 weeks showed about three times more symptom improvement on the GAD-7 (Generalized Anxiety Disorder scale) evaluation compared to the control group that only took their usual medication. Secondary variables, including the PSWQ (Penn State Worry Questionnaire), used to measure mental health, also showed symptom improvement.
Generalized anxiety disorder (GAD) refers to a state where excessive worry and anxiety symptoms persist for more than six months, making it difficult for individuals to control their anxiety. Physical reactions such as muscle tension and palpitations, as well as mental symptoms like restlessness and sleep disturbances, continue, causing significant difficulties in daily life. According to the Health Insurance Review & Assessment Service, as of 2021, the number of anxiety disorder patients in South Korea was approximately 865,000, a 32% increase compared to 2017, and during the same period, depression patients also increased by 35%.
While various causes may contribute to the onset of generalized anxiety disorder, Anzeilax focuses on cognitive distortions such as distorted choices regarding negative environmental factors, distorted information processing, negative views of one’s coping abilities, and inaccurate perceptions of the environment, applying Acceptance & Commitment Therapy to correct these.
Jinwoo Kim, CEO of Hi, introduced, "After the approval of a generalized anxiety disorder digital medical device by the U.S. Food and Drug Administration (FDA) last year, we have launched a product in South Korea that can respond to this global trend and movement."
Jaejin Kim, professor of psychiatry at Gangnam Severance Hospital who led the clinical trial of Anzeilax, said, "This product can help patients suffering from generalized anxiety disorder due to vague anxiety reduce their use of anti-anxiety medication," adding, "If improvements confirmed during the clinical process are supplemented and released, it will help many patients."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.