On-site Investigation Scheduled for the 11th
"We Will Decide on the Disposition After Confirming the Violations"
The Ministry of Food and Drug Safety (MFDS) will conduct an on-site investigation on the 11th into Emart for selling hangover relief products that have not undergone human application trials. This is the first on-site investigation of a violating company since the implementation of the hangover relief product certification system this year.<본지 3월10일자 [단독]이마트, 판매 금지 숙취해소제 '재고떨이' 후폭풍>
An MFDS official stated, "We plan to conduct an on-site investigation at Emart headquarters," adding, "After confirming the violations related to the hangover relief product certification system, we will decide on the disposition and its severity."

According to the hangover relief product certification system implemented from January 1 this year, the sale of hangover relief products using expressions such as 'hangover relief,' 'sobering up,' or 'the day after drinking' without human application trial data is prohibited.
Until now, hangover relief products were classified as general foods and could be sold without human application trials. However, due to criticism over exaggerated advertising and false labeling, the functional labeling system for general foods was introduced on December 29, 2020, followed by a four-year grace period, and has been enforced since this year.
The MFDS anticipated that unintentional violations might occur during the recall of products produced before the system's implementation and decided to operate an additional six-month guidance period. However, it had previously announced that if violations of the obligation to prepare trial data were detected during the guidance period, administrative sanctions would be imposed immediately without further guidance.
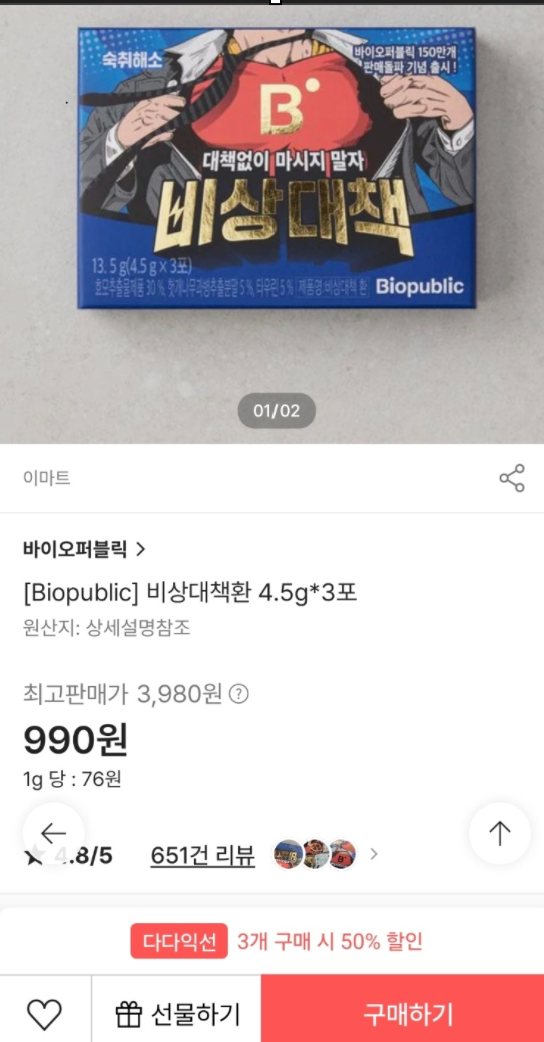
Earlier, Emart sold emergency stock without human application trial data at a drastically discounted price on SSG.com until the 5th. Ahead of the implementation of the hangover relief product certification system this year, Emart had withdrawn the product from offline stores and convenience stores at the end of last year, but its affiliate SSG.com sold it at about 75% less than the regular price of 3,890 KRW. This has raised suspicions that Emart was offloading banned hangover relief products as stock clearance.
The MFDS limited the investigation target to Emart. Emergency stock is Emart’s private brand (PB) health functional food product, manufactured by Inseong Pharmaceutical, with Emart as the specialized distribution seller, and SSG.com as the sales platform. Emart was identified as the party violating the Food Labeling and Advertising Act.
According to Article 8 of the Food Labeling and Advertising Act, failure to comply with the relevant regulations may result in administrative sanctions such as ▲corrective orders ▲recall ▲business suspension ▲product manufacturing suspension.
In the case of the hangover relief product certification system, business suspension is expected immediately upon violation. According to the MFDS’s distributed material titled 'Full Enforcement of Human Application Trial Obligation for Hangover Relief Product Labeling and Advertising,' if human application trial data is not submitted and labeling or advertising continues, a one-month business suspension will be imposed.
Additionally, if the review of trial data finds the hangover relief labeling and advertising inappropriate, a 15-day business suspension will be imposed. If voluntary review is not conducted or the results are not followed, a 15-day product manufacturing suspension will be enforced.
However, business suspension can be replaced by a fine. The basis for calculating the fine is the previous year’s sales. The total sales amount for one year immediately before the sanction is used to impose a fine equivalent to one day of business suspension. Fines replacing product manufacturing suspension are calculated based on the sales performance of the product.
The industry is closely watching the MFDS’s administrative sanctions as the first violation case of the hangover relief product certification system emerges. An industry insider said, "If the sales volume is small, it may end with a corrective order," adding, "However, since there was a grace period of over four years and this is the first detected case, we are watching to see if a heavy sanction will be imposed."
Emart expects a corrective order since it withdrew the product and stopped sales upon recognizing the issue. An Emart representative said, "The product should have been discontinued from this year as it did not undergo separate human application trials, but this occurred due to a misunderstanding of the guidance period," adding, "It is unlikely to escalate to business suspension."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.