UNIST and Hanyang University Research Team Identifies Principle of Electrode Dissolution Caused by Solvent-Cation Interaction
Guidelines Proposed for Electrolyte and Organic Electrode Structure Development; Published in ACS Nano
The cause of the phenomenon where organic battery electrodes dissolve into the electrolyte has been identified.
Professor Wonjin Kwak's team from the Department of Energy Chemical Engineering at UNIST, in collaboration with Professor Junmyung Choi's team from the Department of Mechanical Engineering at Hanyang University, revealed that strong interactions between the solvent and cations occurring inside the electrolyte enhance the dissolution phenomenon of organic battery electrodes.
 Research team. (From right) Researcher Hyunwook Lee (first author), Professor Wonjin Kwak, Researcher Juhyun Lee. Provided by UNIST
Research team. (From right) Researcher Hyunwook Lee (first author), Professor Wonjin Kwak, Researcher Juhyun Lee. Provided by UNIST
Organic batteries are next-generation secondary batteries that replace metal electrodes such as lithium and nickel in commercial batteries with organic materials that are inexpensive and can be mass-produced infinitely in factories.
However, the severe dissolution phenomenon where battery electrodes dissolve into the electrolyte results in a short battery lifespan, which is a major obstacle to commercialization. Although various studies have been reported to solve this issue, the exact cause of dissolution had not been clearly identified.
According to the research results, strong interactions between the solvent and cations cause co-intercalation. The electrolyte consists of anions and cations dissolved in a liquid solvent, and co-intercalation is the phenomenon where solvent molecules are dragged along when cations enter the microstructure inside the electrode. When the solvent is dragged in, the microstructure inside the electrode material expands, causing the electrode material to easily flow out. When the interaction is weak, normal intercalation occurs where only cations enter the electrode.
The research team identified this fact by comparing and analyzing experimental results where the type of cation was changed and theoretical calculations of the interaction energy between the solvent and cations. When experiments were conducted by changing lithium, sodium, and potassium ions, the electrode thickness was thinnest when lithium ions were used, and the interaction energy between cations and solvent molecules was also the greatest.
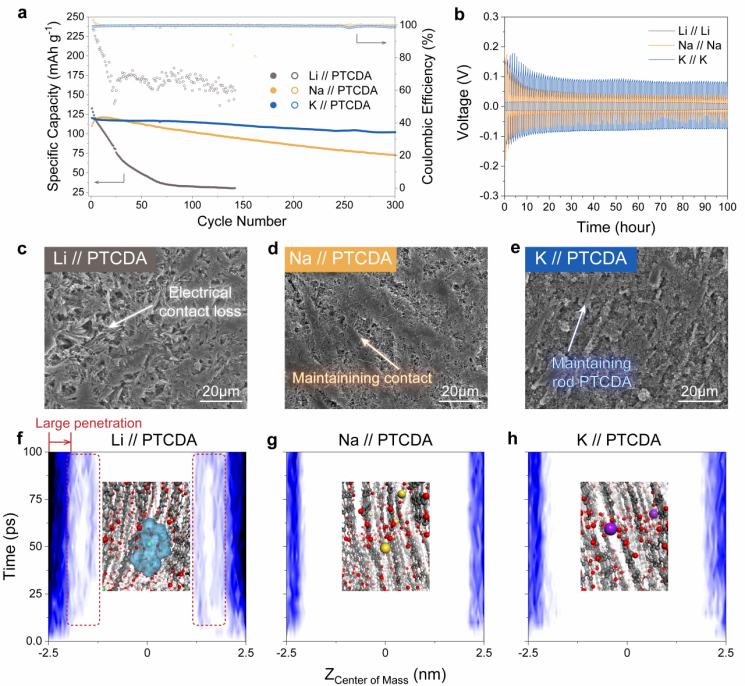 Performance and Co-insertion Differences of Electrodes by Type of Cation in Electrolyte. Provided by UNIST
Performance and Co-insertion Differences of Electrodes by Type of Cation in Electrolyte. Provided by UNIST
Hyunwook Lee, the first author of the study, explained, “Previous research related to organic electrodes mainly focused on solving the dissolution phenomenon through changes in electrode materials or structures, but this study is meaningful in that it identified the fundamental cause of dissolution.”
Professor Wonjin Kwak said, “Through this study, we confirmed for the first time that electrode material dissolution is not simply due to solubility but is caused by interactions within the electrolyte and the resulting changes in mechanisms, and we proposed specific electrolyte design strategies.”
The research results were published on January 14 in ACS Nano, a leading international journal in the field of nanoscience. The research was supported by the Nano and Materials Technology Development Project of the Ministry of Science and ICT.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















