Briefing Session on the Regenerative Medicine Treatment System
for Regenerative Medical Institutions and Cell Processing Facilities
Hosted by the Ministry of Health and Welfare
The Ministry of Health and Welfare announced that, in accordance with the revision of the "Act on the Safety and Support of Advanced Regenerative Medicine and Advanced Biopharmaceuticals (Advanced Regenerative Bio Act)," a new advanced regenerative medicine treatment system will be introduced and implemented starting from the 21st.
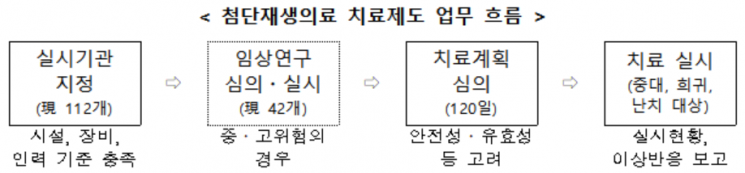
Regenerative medicine technology refers to cell therapy, gene therapy, tissue engineering therapy, and other treatments conducted using human cells. The advanced regenerative medicine treatment system is a system that allows regenerative medicine technologies, which have been verified through prior medium- to high-risk clinical research, to be used for the treatment of serious, rare, and intractable diseases after review by the Advanced Regenerative Bio Deliberation Committee composed of experts. It was established to provide new treatment opportunities for patients with rare and intractable diseases who have no alternative treatments, and to support the development of regenerative medicine technology by leading to pharmaceutical approvals based on analysis and evaluation of treatment outcomes.
Medical institutions wishing to conduct advanced regenerative medicine treatments must first meet facility, equipment, and personnel requirements in advance and receive designation from the Minister of Health and Welfare. After a medical institution is designated as a conducting institution, it must submit materials such as the purpose and target of the treatment to be conducted, evidence of safety and efficacy, and cost calculation basis to the Deliberation Committee to receive a review on the appropriateness of the treatment plan.
If the treatment plan receives an appropriate review, treatment can be conducted for the period specified in the plan. At this time, regenerative medical institutions must comply with implementation standards such as adhering to the reviewed treatment plan, preparing and following measures in case of adverse reactions, and confirming the health status of treatment subjects.
In line with the implementation of the system, the Ministry of Health and Welfare held a "Joint Briefing Session of Related Ministries on the Regenerative Medicine Treatment System" on the 19th at the Korea Press Center in Jung-gu, Seoul, with participation from the Ministry of Food and Drug Safety, the Korea Disease Control and Prevention Agency’s National Institute of Health, the Deliberation Committee Secretariat, and the Regenerative Medicine Promotion Foundation, to provide guidance on treatment execution procedures and preparation matters to regenerative medical institutions and cell processing facilities.
Shin Kkotshigye, Advanced Medical Support Officer at the Ministry of Health and Welfare, stated, "Through the advanced regenerative medicine treatment system, we expect to provide new treatment opportunities to patients who have not been able to receive cell therapy and other treatments domestically so far," and added, "We will organize and strengthen the review and management system to ensure that patients can receive treatment safely."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.