Rare genetic disease diagnosis company Three Billion announced on the 26th that it has been selected as a support target for the LA region under the overseas branch establishment project organized by the Korea Trade-Investment Promotion Agency (KOTRA).
This project aims to support the business expansion of domestic companies with high growth potential in overseas markets by assisting in building business foundations and forming networks locally abroad.
Three Billion will promote the branch establishment project centered on the LA area in the United States for the next year with KOTRA's support. During the branch establishment process, the company plans to focus on discovering local potential buyers, localizing products and services, and building local infrastructure to expand its network in the North American market. The goal is to get closer to rare disease patients within the North American market.
Rare diseases are 80% genetic disorders, and patients occur proportionally to the population. The metropolitan area surrounding LA has a population of 18 million, making it the second most populous region in the United States. It also has the second highest number of rare disease patients in the country. This makes it a suitable region for Three Billion’s initial business in the U.S.
Three Billion secured the California Clinical Laboratory certification, CDPH certification, in April 2023, qualifying it to provide tests covered by U.S. health insurance for patients residing in California. The selection for the KOTRA project is expected to accelerate Three Billion’s business expansion in the LA region.
Dr. Lee Suk-jin, Chief Business Officer (CBO) of Three Billion, said, "Through KOTRA’s overseas branch establishment project, we have gained an opportunity to enter the local market more deeply," adding, "We plan to build closer relationships with potential customers and partners in the U.S. through rapid market entry and product localization."
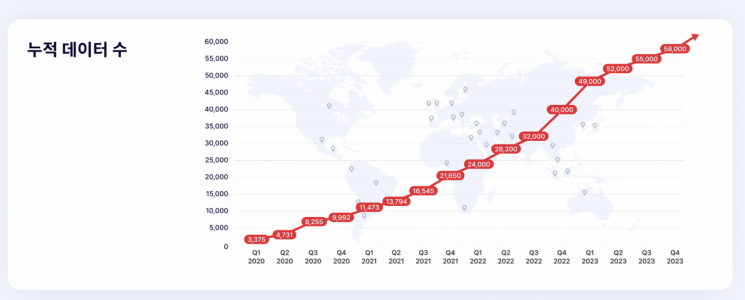
Three Billion has already collaborated with over 700 institutions in more than 70 countries, accumulating genomic data of approximately 70,000 rare disease patients. It was listed on the KOSDAQ market on the 14th, recognized for its industry-leading technology and potential marketability. Its overseas sales account for as much as 70%, showing rapid growth in overseas markets.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















