It has been over three years since the immune checkpoint inhibitor Imfinzi was approved as a first-line treatment for cholangiocarcinoma, but it still has not been covered by the National Health Insurance, prompting calls for its rapid inclusion during this year's National Assembly audit.
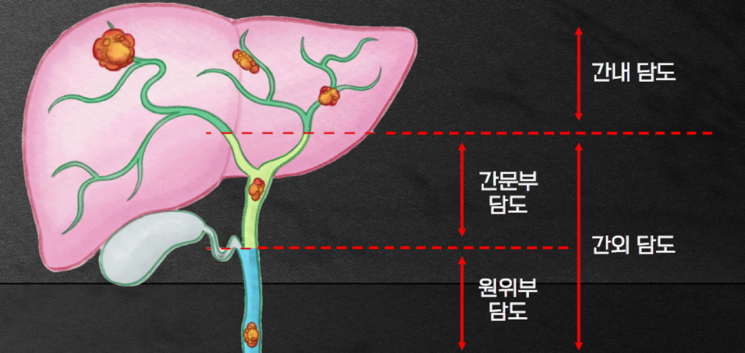
 Cholangiocarcinoma is known to have a poor prognosis due to its organ characteristics, resembling thin branches and spanning the liver and duodenum. Moreover, since there are no symptoms that can raise suspicion of cancer in the early stages, 7 out of 10 patients with cholangiocarcinoma are diagnosed with cancer at an advanced stage, where surgery is no longer possible or metastasis has occurred.
Cholangiocarcinoma is known to have a poor prognosis due to its organ characteristics, resembling thin branches and spanning the liver and duodenum. Moreover, since there are no symptoms that can raise suspicion of cancer in the early stages, 7 out of 10 patients with cholangiocarcinoma are diagnosed with cancer at an advanced stage, where surgery is no longer possible or metastasis has occurred. [Image source=Getty Images]
Seo Myeong-ok, a member of the National Assembly's Health and Welfare Committee from the People Power Party, recently submitted a written inquiry to the Health Insurance Review and Assessment Service (HIRA) during the National Assembly audit, stating, "There is an urgent need for health insurance coverage of this new drug that has doubled the survival rate of cholangiocarcinoma, a disease often considered a death sentence," and asked what efforts are being made to improve access to new drugs for cholangiocarcinoma.
Cholangiocarcinoma is known to have a poor prognosis due to the anatomical characteristics of the bile ducts, which resemble thin branches extending across the liver and duodenum. Moreover, since there are no symptoms that raise suspicion of cancer in the early stages, 7 out of 10 cholangiocarcinoma patients are diagnosed at an advanced stage where surgery is no longer possible or the cancer has metastasized.
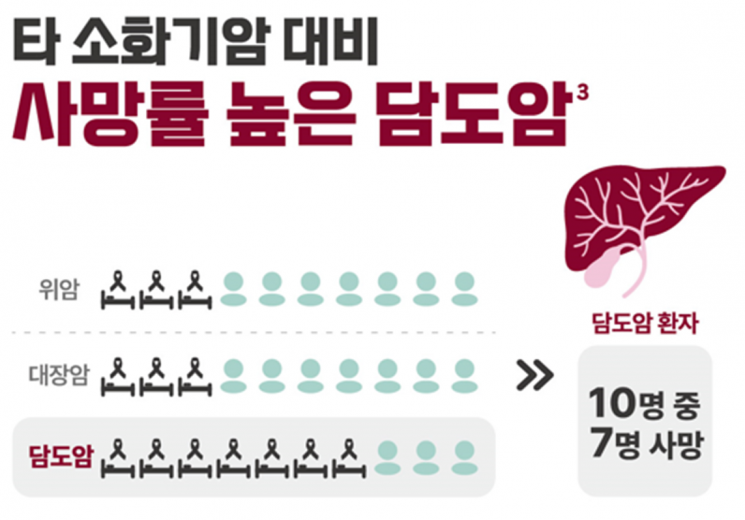
As a result, unlike other cancers in Korea where treatment environments are steadily improving and patient survival rates are rapidly increasing, the mortality rate for cholangiocarcinoma remains among the lowest worldwide. Korea has the highest cholangiocarcinoma mortality rate globally, with 11.64 deaths per 100,000 patients. The 5-year relative survival rate (the survival rate of cancer patients compared to the general population) is only 28.9%, about one-third of the 72.1% average for all cancer patients.
However, recent developments in immunotherapy have raised hopes of extending patients' lives. Imfinzi, an immune checkpoint inhibitor developed by AstraZeneca (AZ), is the first new treatment option for cholangiocarcinoma in 12 years, previously treated only with chemotherapy (gemcitabine and cisplatin combination therapy).
In the Phase 3 clinical trial 'TOPAZ-1,' adding Imfinzi to standard chemotherapy resulted in an overall survival rate of 24.9% at two years, 1.5 times higher than the 10.4% survival rate with chemotherapy alone. The recently released 3-year overall survival follow-up data confirmed a more than twofold difference, with 14.6% versus 6.9%. Notably, a subgroup analysis of Korean patients participating in the trial showed an even better effect, with a 3-year survival rate of 21.0% compared to 14.6%.
Based on these results, Imfinzi received approval from the U.S. Food and Drug Administration (FDA) in September 2022 and was subsequently approved by Korea's Ministry of Food and Drug Safety in November as a combination therapy for cholangiocarcinoma. It also received approval last June for dual immunotherapy with another immune checkpoint inhibitor, Imjudo, for liver cancer. However, the inclusion of Imfinzi in the National Health Insurance coverage has faced difficulties. Although coverage was applied for last year for cholangiocarcinoma, only chemotherapy was covered despite Imfinzi being part of the combination therapy, leaving patients to bear the high cost of Imfinzi out-of-pocket. Korean AZ reapplied for coverage review for both cholangiocarcinoma and liver cancer indications in June.
Seo Myeong-ok's inquiry highlights the need for HIRA's commitment to improving access to new drugs for cholangiocarcinoma under these circumstances. In response, HIRA stated, "The combination therapy with Imfinzi was decided to be fully paid by patients due to the high cost and significant financial burden, as determined by the Cancer Disease Deliberation Committee held in November last year," adding, "If the pharmaceutical company submits additional data, we will promptly review it according to procedures."
In response, AstraZeneca Korea said, "We are doing our best to provide innovative treatment options to cholangiocarcinoma patients in Korea," and added, "We will prioritize patients and families waiting for Imfinzi coverage and strive to pass the cancer disease deliberation within this year. We also hope the government empathizes with the urgent voices of cholangiocarcinoma patients and their families."
Recently, voices from patients urging for rapid Imfinzi coverage have been growing louder, including a public petition submitted by a caregiver of a cholangiocarcinoma patient citing economic reasons for the urgent need for coverage.
A petitioner, who identified themselves as the child of a 55-year-old patient with stage 4 intrahepatic cholangiocarcinoma, recently submitted a petition through the National Assembly's public petition system requesting the rapid inclusion of Imfinzi in insurance coverage. They emphasized that "Imfinzi saved my mother," and said, "My mother is surviving healthily with Imfinzi treatment, but the monthly treatment cost of 10 million KRW is a heavy burden," appealing, "Imfinzi coverage is urgently needed so that my mother and other cholangiocarcinoma patients in Korea can survive healthily for a long time."
The petition received about 8,000 signatures. Although it did not meet the 50,000-signature threshold required for official public petitions, it is regarded as a meaningful social awakening for cholangiocarcinoma patients, whose prevalence is less than a quarter of that of major cancers like breast cancer and whose awareness is relatively low.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.