MFDS Introduces and Supplies mRNA Vaccines for Ages 6 Months to 4 Years
The Ministry of Food and Drug Safety announced on the 21st that it has granted emergency use authorization for Pfizer's Comirnaty JN1 injection 0.033 mg/mL (active ingredient Bretobameran), a COVID-19 variant (JN.1) vaccine for infants and young children aged 6 months to 4 years.
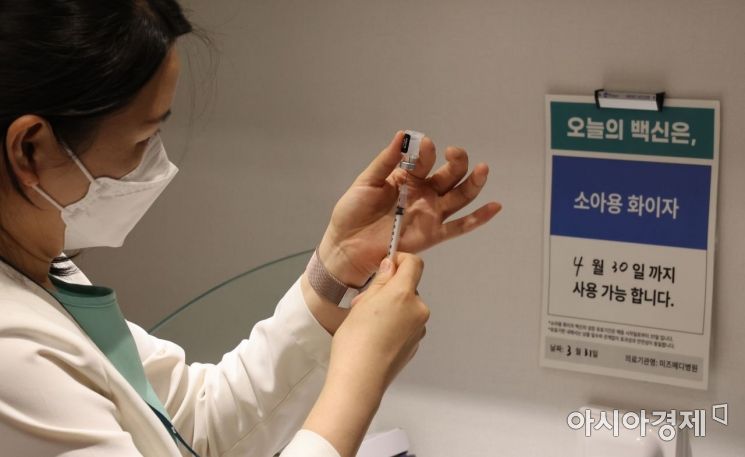 On the 31st, when COVID-19 vaccination with Pfizer began for children aged 5 to 11, medical staff were preparing the vaccine at the Department of Pediatrics and Adolescents at Mizmedi Hospital in Gangseo-gu, Seoul. Photo by Joint Press Corps
On the 31st, when COVID-19 vaccination with Pfizer began for children aged 5 to 11, medical staff were preparing the vaccine at the Department of Pediatrics and Adolescents at Mizmedi Hospital in Gangseo-gu, Seoul. Photo by Joint Press Corps
Emergency use authorization is a system that allows the Minister of Food and Drug Safety to permit manufacturers or importers to produce or import medical products not approved domestically upon request from the heads of relevant central administrative agencies, in order to appropriately respond to public health crises such as infectious disease pandemics.
The Korea Disease Control and Prevention Agency requested emergency use authorization for this vaccine to vaccinate infants and young children, and the Ministry of Food and Drug Safety reviewed and approved it promptly.
Previously, the Ministry approved Pfizer Korea's Comirnaty JN1 injection and Moderna Korea's Spikevax JN injection as JN.1 variant vaccines for those aged 12 and older, and granted emergency use authorization for the Novavax COVID-19 vaccine (2024-2025 formulation).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















