Seoul National University Research Team Confirms 'USF2'-'TFEB' Interaction Mechanism
Increases Potential for Removing Alzheimer's Causative Proteins
A mechanism in which the transcription factor TFEB (transcription factor EB), which activates intracellular autophagy, and the transcription factor USF2 (upstream transcription factor 2), which inhibits it, compete and interact with each other has been revealed for the first time by a domestic research team. This discovery draws attention as it may open a new path to treat neurodegenerative diseases such as Alzheimer's and Parkinson's, caused by the increase of beta-amyloid protein or tau protein accumulation in brain cells.
 Professor Baek Seonghee and Researcher Kim Jaebeom, Department of Biological Sciences, Seoul National University
Professor Baek Seonghee and Researcher Kim Jaebeom, Department of Biological Sciences, Seoul National University
On the 14th, Jaebeom Kim, a doctoral researcher in Professor Seonghee Baek’s team at the Department of Biological Sciences, Seoul National University, announced that they identified the pathway through which USF2 and TFEB mutually influence the expression of autophagy and lysosomal genes. Although the possibility that USF2 could be involved in autophagy had been reported before, this is the first confirmation that it interacts with TFEB and functions differently depending on the cell’s nutritional status.
Autophagy is one of the body's self-cleaning processes that maintains cellular homeostasis by removing damaged or aged organelles within cells. It is known to significantly affect weight loss, youthfulness, and the maintenance of a healthy brain, prompting various studies to elucidate its operating mechanisms.
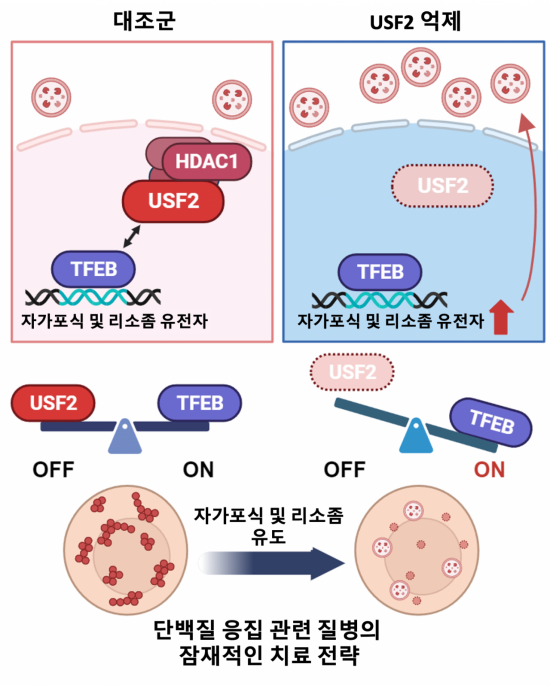
The research team created a mouse model deficient in USF2 using gene editing tools and produced primary cell lines for molecular biological analysis. They found that in nutrient-rich environments, USF2 recruits the NuRD (Nucleus Remodeling Deacetylase) complex to epigenetically suppress the expression of autophagy genes and inhibit unnecessary autophagic activity. Conversely, under nutrient-deprived conditions, TFEB activates the autophagy pathway, playing a crucial role in cell survival. Notably, in cases of nutrient deficiency, dephosphorylation of USF2 reduces its DNA-binding affinity, allowing TFEB to act more effectively. This represents an important regulatory mechanism for cell survival under stress conditions.
It was also discovered that USF2 competitively binds to the same sites on autophagy and lysosomal genes as TFEB, establishing a balance between the two transcription factors. The research team explained that this competitive mechanism serves as a key factor in determining the gene expression patterns of cells and regulates the activation or suppression of the autophagy pathway.
The team confirmed through an 'alpha-1 antitrypsin deficiency disease' model that inhibiting USF2 promotes autophagy. Since autophagy activation reduces protein aggregation, this finding demonstrates its potential application in treating diseases caused by protein aggregation.
Researcher Kim stated, "This important discovery using USF2 could present new therapeutic strategies for protein aggregation diseases such as lysosomal storage disorders and neurodegenerative diseases," adding, "Furthermore, it could contribute to the development of personalized treatments based on gene regulation mechanisms."
The research results were published on the 27th of last month in the international scientific journal Nature Communications.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.