Declined Due to 2022 Pandemic Base Effect
5-Year Growth Trend Excluding Korota Special Products
Selected 46 High-Potential Items for "Export Support"
South Korea's medical device export value has increased by an average of more than 8% annually over the past five years since 2019, according to an analysis.
The Ministry of Trade, Industry and Energy and the KOTRA Trade and Investment Research Center announced on the 4th that they published a report titled "Endemic Era, Medical Device Strategic Items and Market Analysis" containing this information.
Medical device industry exports recorded a record high of $9.22 billion (approximately 12.64 trillion KRW) in 2021. Last year, exports decreased by 37% to $5.79 billion (approximately 7.94 trillion KRW). This reflects the export performance of in vitro diagnostic devices, which benefited from the pandemic boom in 2020 and 2021.
Excluding in vitro diagnostic devices, medical device exports amounted to $5 billion (approximately 6.85 trillion KRW) last year. Since recording $3.6 billion (approximately 4.935 trillion KRW) in 2019, the compound annual growth rate over five years was 8.4%. Laser devices, implants, ultrasound imaging diagnostic devices, and dental X-ray devices led the export expansion.
According to the report, among the top 10 medical device export-leading items, small and medium-sized enterprises (SMEs) and mid-sized companies accounted for more than 80% in nine items. After the pandemic, the export destination countries diversified. The share of exports to China decreased from 15% to 11%. Emerging markets such as Brazil, Russia, and India replaced this share.
Jung Sun-young, head of the center, said, "Recently, products such as implants and dental X-ray devices have shown competitiveness in the export market," adding, "It is necessary to carefully select products with high growth potential in terms of securing new growth engines and expanding exports and nurture them as next-generation export-leading items."
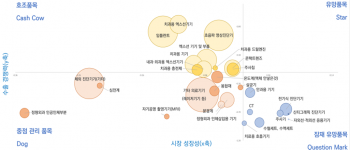
The center selected 46 items with high potential after analyzing export statistics by medical device industry category. They were classified into three groups: favorable, promising, and priority management items.
Favorable items are those with good export status but require strengthening competitiveness through ultra-gap technology development in preparation for intensified future competition. These include implants, X-ray device components, dental X-ray devices, dental devices, and contact lenses.
Promising items have high growth potential but need improvement in export competitiveness. Selected items include electric diagnostic devices (body composition analyzers, patient monitoring devices, blood pressure monitors), ophthalmic devices, catheters and cannulas, syringes, and computed tomography (CT) scanners. Mid- to long-term research and development (R&D) investment and support for new market development are necessary.
Priority management items are those expected to face intensified competition or decreased global demand in the future, requiring efforts such as creating alternative demand and discovering substitute items. These include other medical devices (pregnancy test kits, endoscopes, laser devices, etc.) and in vitro diagnostic devices.
The center plans to publish the fourth part of the report this month, analyzing export competition trends and promising markets for the top 12 export items among these. The report can be found on KOTRA Overseas Market News.
KOTRA's Bio-Medical Team has established 'K-Bio Desks' in seven regions, including the United States and Germany, to support domestic biohealth companies' export sites. They provide support for export difficulties such as local certification, customs clearance, and logistics, including CE-MDR (European Union Medical Device Regulation) certification consulting, and are expanding specialized export support projects.
Lee Ji-hyung, head of KOTRA's Economic and Trade Cooperation Headquarters, said, "Medical devices are a field attracting attention as a future growth engine with rapid growth speed," adding, "KOTRA will solidly promote export support projects so that 46 medical device items become main export items through local inspections by overseas trade centers and cooperation with related ministries and agencies."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.