Prevention of Ionomer Degradation and Oxidation Improves Hydrogen Production Efficiency
Low-Cost Catalyst Enhances Both Performance and Durability of Hydrogen Production Devices
The principle that can prevent the degradation and oxidation of ionomers occurring in the anion exchange membrane method for hydrogen production has been revealed for the first time.
This is expected to simultaneously improve the performance and durability of hydrogen production devices.
A research team led by Professor Seunggeol Lee from the Department of Materials Science and Engineering at UNIST (President Jongrae Park) has proposed a new anion exchange membrane water electrolysis technology using low-cost non-platinum metal catalysts.
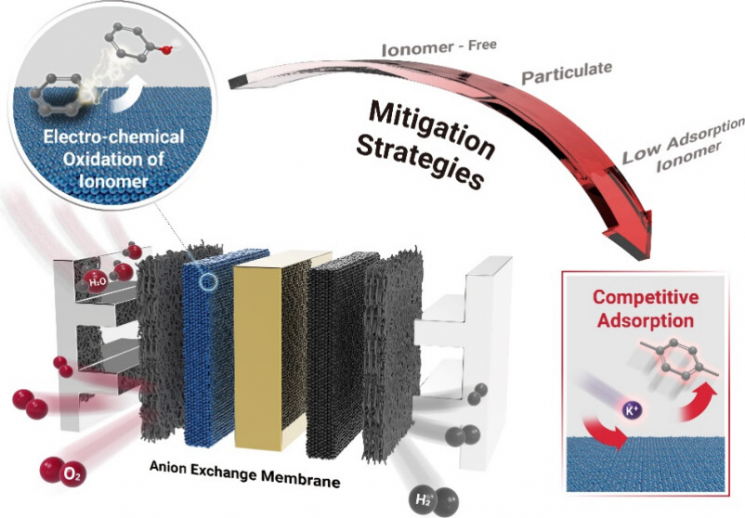
The method involves attaching potassium to the catalyst surface to reduce direct contact with the ionomer. By preventing the oxidation of the ionomer, it is possible to reduce the cost of hydrogen production.
In general hydrogen production devices, the properties of the ionomer, which delivers ionic substances, tend to weaken over time. This has resulted in reduced hydrogen production efficiency and shortened device lifespan.
The research team utilized the fact that the adsorption energy of potassium is more than three times greater than that of organic compounds. They found that substances such as potassium hydroxide and sodium hydroxide can enhance the performance and stability of anion exchange membrane water electrolysis systems.
Cationic substances adsorbed onto the catalyst surface reduced direct contact between the ionomer and the catalyst. Ultimately, they proved through density functional theory (DFT) calculations of electronic structures that this prevents ionomer oxidation and maintains hydrogen production performance.
Although there have been previous attempts to improve performance using strongly alkaline potassium hydroxide and sodium hydroxide solutions, the specific principle had not been revealed. However, the competitive adsorption strategy identified in this study is expected to further increase the commercialization potential of low-cost catalysts.
First author Jihoon Lim, a researcher, emphasized, "The competitive adsorption strategy is effective in reducing the electrochemical oxidation of ionomer materials occurring at the interface with the catalyst."
Professor Seunggeol Lee said, "This study will provide direction for improving the performance and stability of various energy devices, including high-performance alkaline anion exchange membrane water electrolysis systems."
The research results were published online on June 2 in ACS Energy Letters, a leading academic journal in the energy field. The research was conducted in collaboration with Dr. Yu Seung Kim's team at Los Alamos National Laboratory in the United States, Professor Shannon Boettcher at the University of California, Berkeley and Berkeley Lab, and was supported by the U.S. Department of Energy and the National Research Foundation of Korea.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)










