Lunit Accelerates Entry with Bolpara Acquisition
Vuno Expects Approval and Launch of 'DeepCAS' Within the Year
Coreline, JLK, Neurofit Also Prepare for Market Entry
Domestic medical artificial intelligence (AI) companies are accelerating their entry into the U.S. market.
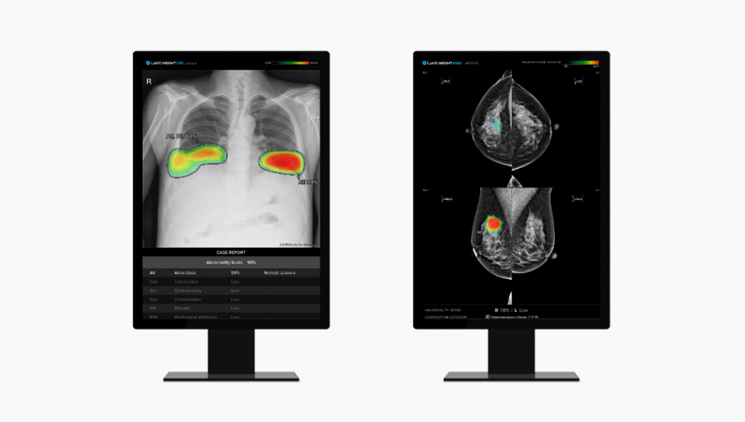 Lunit's chest X-ray AI imaging analysis solution 'Lunit Insight CXR' (left) and mammography AI imaging analysis solution 'Lunit Insight MMG'. [Photo by Lunit]
Lunit's chest X-ray AI imaging analysis solution 'Lunit Insight CXR' (left) and mammography AI imaging analysis solution 'Lunit Insight MMG'. [Photo by Lunit]
AI imaging inspection solution company Lunit announced that the acquisition of Volpara Health Technologies, which will serve as a foothold for its entry into the U.S. market, is in its final stages. Volpara, a New Zealand company that Lunit announced its acquisition of last December, has a solid sales network in the U.S., supplying products such as AI solutions for mammography to 42% of U.S. medical institutions. Lunit’s main products are AI imaging inspection solutions, and Volpara is expected to become the hands and feet of Lunit’s U.S. market strategy.
Lunit plans to complete the acquisition of Volpara by next month and accelerate the market entry of its three main products?Lunit Insight CXR Triage, Lunit Insight MMG, and Lunit Insight DBT?which have already started sales in the U.S. CXR Triage classifies emergency diseases through chest X-ray imaging, while MMG and DBT are auxiliary solutions for breast cancer screening.
What Lunit emphasizes in its U.S. market entry is its technological prowess. A Lunit representative said, "We continue to publish numerous papers at major global conferences to demonstrate the accuracy of our technology." Lunit presented seven research results at the American Association for Cancer Research (AACR) held earlier this month. In this process, they also produced results proving the practical use of medical AI. Through recent collaboration with Turkish researchers, Lunit confirmed that using Lunit Insight MMG reduces medical staff workload by 70% and improves classification accuracy by 30%. This demonstrates that AI can be a solution to alleviate doctors’ excessive workload amid the recent medical crisis.
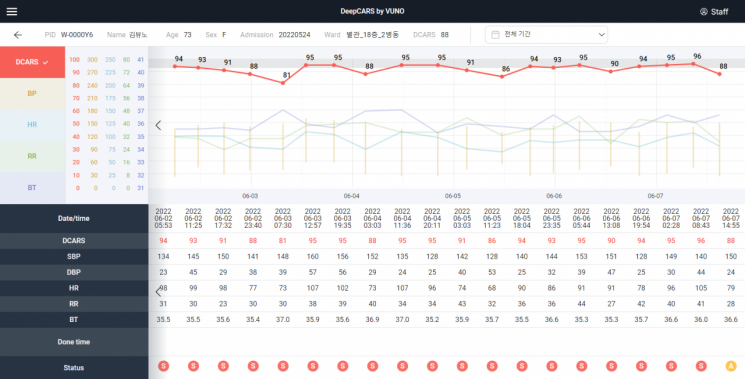 Operating screen of Vuno's AI-based cardiac arrest prediction medical device 'Vuno Med DeepCARS'
Operating screen of Vuno's AI-based cardiac arrest prediction medical device 'Vuno Med DeepCARS' [Photo by Vuno]
According to market research firm Prescience Research, the global AI healthcare market is expected to grow rapidly at an average annual rate of 37%, from $15.1 billion (approximately 21 trillion KRW) in 2022 to $187.95 billion (approximately 260 trillion KRW) by 2030. The U.S. is known to hold about 60% of this market share. Besides Lunit, other domestic medical AI companies such as Vuno, Coreline Soft, JLK, and Neurofit are hastening preparations for U.S. entry, including obtaining approval from the U.S. Food and Drug Administration (FDA).
Vuno recently completed the U.S. trademark registration for VunoMed DeepCARS, an AI-based cardiac arrest prediction medical device. It was designated as a breakthrough device by the U.S. FDA in June last year. The company expects FDA approval and launch within the year, stating, "Being designated as a breakthrough device allows close support from the FDA during the approval process and prioritizes the approval procedure."
VunoMed DeepCARS analyzes vital signs such as respiration, blood pressure, pulse, and body temperature of general ward inpatients to predict the risk of cardiac arrest within 24 hours. As of last month, it has been adopted by a total of 83 hospitals, including 15 tertiary general hospitals, and is being trial-operated in about 40 hospitals, establishing its presence.
Coreline Soft received premarket approval from the FDA on the 2nd for Aview CAC, an AI-based atherosclerosis diagnostic support solution. It helps quickly assess the level of coronary artery calcification, an indicator of atherosclerosis, through assistance in interpreting heart or chest CT scans. This approval increases the number of FDA-approved products to nine. Coreline Soft is currently selling lung cancer screening solutions in the U.S.
JLK is knocking on the U.S. door with an AI solution that quickly assists in classifying types of cerebral infarction. They are confident that clinical papers have proven superior efficacy compared to products already released in the U.S. They plan to apply for FDA approval for a total of five AI imaging analysis solutions within this year. Neurofit is also preparing to enter the U.S. market after receiving FDA approval for solutions that rapidly assist in interpreting positron emission tomography (PET) and magnetic resonance imaging (MRI) related to brain diseases.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















