HLB's 'Rivoceranib' and Yuhan's 'Reclaza'
Expected Approval Within the Year... Anticipation for Launch Within the Year
Last Year's Approved Zympenetra and Aliglo Also Making Rapid Progress Toward Launch
The number of domestic new drugs receiving approval from the U.S. Food and Drug Administration (FDA), the entry permit for entering the U.S. market?the world's largest pharmaceutical market?is rapidly increasing. Since plans are in place to proceed with the U.S. launch immediately upon approval, tangible results are also expected.
According to HLB on the 2nd, the final review process for the liver cancer treatment drug, Lenvatinib, which is undergoing FDA new drug approval procedures, was recently completed. Jin Yang-gon, chairman of HLB, declared at last month's shareholders' meeting, "The final review meeting ended on a positive note," adding, "There were no issues from the first review, so we have been confident since then."
HLB's oral anticancer drug, Lenvatinib, is knocking on the door of FDA approval through combination therapy with Camrelizumab, an immune checkpoint inhibitor developed by China's Hansoh Pharmaceutical. This therapy extended the median overall survival (OS)?the period from treatment initiation to patient death?to 22.1 months in phase 3 clinical trials. Compared to the existing standard treatment's overall survival period of 12 to 13 months, this result significantly increased patients' expected lifespan.
Similarly, Yuhan Corporation's lung cancer treatment drug, Lekraza, which is also aiming for FDA approval through combination therapy, is expected to have its approval decision by August. Lekraza is preparing for approval through combination therapy with Johnson & Johnson's (J&J) targeted antibody treatment, Rybrevant, to which the technology was licensed out.
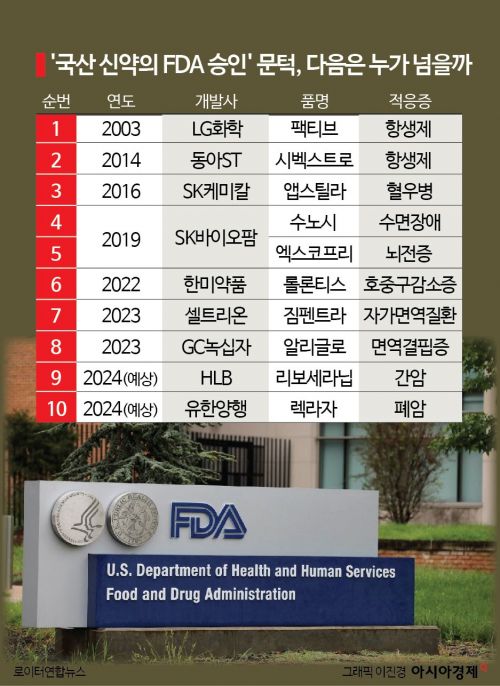
These two drugs are attracting attention as the first anticancer drugs among FDA-approved domestic new drugs. Existing FDA-approved domestic new drugs cover various diseases such as autoimmune diseases, epilepsy, and hemophilia, but the only anticancer-related drug was Hanmi Pharmaceutical's Rolontis. Rolontis is a drug that treats neutropenia, a side effect occurring during cancer treatment, rather than being a fundamental anticancer agent.
Meanwhile, HLB and Yuhan Corporation plan to launch their drugs in the U.S. market immediately after approval. HLB has already set the launch date for Lenvatinib as September 3, about four months after the notification deadline for approval. Chairman Jin said, "This date means the day when delivery operations are carried out so that prescriptions can be made after launch," adding, "Since January last year, we have formed a marketing team and started sales." HLB plans to sell Lenvatinib and Camrelizumab directly through its U.S. subsidiary, Eleva.
Lekraza is also expected to be launched within the year. Geun-hee Seo, a researcher at Samsung Securities, explained, "Fast U.S. launch is expected due to priority review approval," and "FDA approval of the combination therapy is expected by August, with U.S. market release in the fourth quarter." Especially, since J&J, a multinational pharmaceutical company with strong sales capabilities, will handle direct sales, rapid growth is anticipated.
Earlier, other domestic new drugs approved last year are also accelerating their entry into the U.S. market. Celltrion's autoimmune disease treatment new drug, Zimpentra, which received FDA approval in October last year, was launched in the U.S. last month.
The active ingredient of Zimpentra, infliximab, has been on the market for 26 years but still holds a market share in the inflammatory bowel disease field in the U.S. However, since only intravenous injection products requiring visits to medical institutions were available, there was a significant unmet demand in the U.S. for a subcutaneous injection formulation that patients can administer at home. Zimpentra plans to leverage its advantage as the only subcutaneous injection formulation among infliximab treatments to target the U.S. market. Celltrion expects sales of 500 billion to 600 billion KRW in the U.S. alone this year.
GC Green Cross's blood product for congenital immunodeficiency treatment, Aliglod, which was approved in December last year, is set to launch in July. Unlike existing treatments, which had concerns about thromboembolism side effects caused by blood clotting in blood vessels, Aliglod significantly improves safety by removing 99.9% of the triggering factors.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.