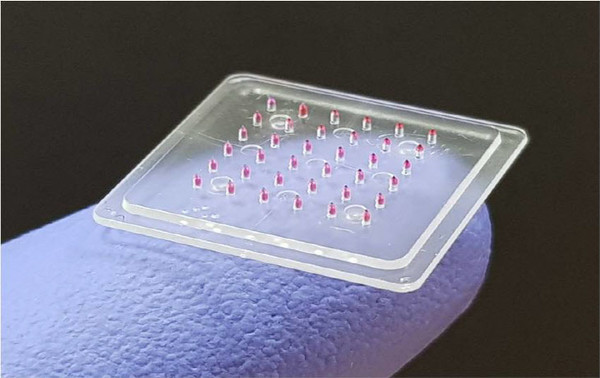
Daewon Pharmaceutical announced on the 6th that the clinical phase 1 trial plan (IND) for DW-1022, a microneedle patch obesity treatment jointly developed with Lapas, has been approved.
DW-1022 is a microneedle-type patch containing semaglutide, a diabetes and obesity treatment ingredient developed by Novo Nordisk. Daewon Pharmaceutical and Lapas are developing the ingredient used in the injectable obesity treatment Wegovy into a patch form that is applied to the skin.
In this phase 1 clinical trial, the safety and pharmacokinetic properties of DW-1022 will be evaluated in healthy adult volunteers, and the relative bioavailability will be assessed using Wegovy as a comparator. A total of 30 participants will be enrolled, with three different doses administered once each, increasing the dose stepwise. The clinical trial is scheduled to be completed by November, and the results are expected to be available within the year.
Semaglutide and other glucagon-like peptide (GLP)-1 receptor agonists, which have recently led the obesity treatment market, are mostly peptides composed of linked amino acids. Therefore, their bioavailability is very low when administered orally, making it difficult to expect therapeutic effects. Daewon Pharmaceutical and Lapas have improved medication convenience by eliminating the hassle and pain of self-injection, while concentrating the drug on the advanced part of the microneedles to minimize waste of the active pharmaceutical ingredient. The product also has the advantage of being easier to store at room temperature compared to existing injectables, which is expected to make distribution more convenient.
A Daewon Pharmaceutical official stated, "DW-1022 uses microneedles less than 1 mm in length to achieve high delivery efficiency, offering a new option besides injectables and oral drugs," adding, "We will proceed with the clinical trial as planned without any setbacks."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)













