Vaccines that were originally administered to prevent diseases are now transforming into therapeutic agents. The idea is that if vaccines can boost immunity like traditional ones, they can also be used to treat cancer and prevent its recurrence. With market growth anticipated, various companies both domestically and internationally are stepping up to develop these therapies.
Generally, vaccines are given before a disease occurs to prevent its onset. For example, cervical cancer can be caused by the human papillomavirus (HPV), so there are cancer prevention vaccines like MSD’s Gardasil. However, most newly developed cancer vaccines aim to be 'therapeutic vaccines' that enhance the immunity of patients already diagnosed with cancer, treating the cancer and training immune cells to remember and attack cancer cells to prevent recurrence.
So far, the only approved cancer therapeutic vaccine is Provenge, developed by the US company Dendreon. It received FDA approval in 2010 for prostate cancer. However, commercialization faced difficulties, leading Dendreon to the brink of bankruptcy, and the cancer therapeutic vaccine market did not grow properly afterward.
However, with the experience of COVID-19, various vaccine platforms such as messenger RNA (mRNA), DNA, and peptides have emerged, rekindling expectations. The Korea Health Industry Development Institute forecasts that the global cancer therapeutic vaccine market will grow from $345.63 million (approximately 450 billion KRW) in 2020 to $897.02 million (approximately 1.2 trillion KRW) by 2027, at an average annual growth rate of 14.6%. Although the preventive vaccine market, which is already commercialized, is larger, therapeutic vaccines are growing faster and are expected to increase their share within the total cancer vaccine market, which is projected to reach 32 trillion KRW by 2027.
In particular, in Korea, the cancer therapeutic vaccine market is expected to grow at an average annual rate of 16.0%, steeper than the global average. Accordingly, various domestic companies are challenging the development of therapeutic vaccines. Recently, DXVX signed a letter of agreement on key transaction terms to introduce the anticancer vaccine OVM-200, developed by its affiliate Oxford Vacmedix in the UK. Phase 1 clinical trials are underway to confirm immune efficacy and safety, as well as to observe actual anticancer effects in patients with ovarian, prostate, and lung cancers.
Among other domestic companies, Aston Science is considered the most advanced. Their DNA-based breast and stomach cancer therapeutic vaccine AST-301 is currently in global Phase 2 clinical trials. It was reported that long-term safety and immunogenicity were confirmed in Phase 1. In addition, four pipelines?AST-201 (ovarian cancer), AST-302 (breast cancer), and AST-021p (solid tumors)?have entered clinical trials in humans. Furthermore, Hanmi Pharmaceutical is developing HM99462, targeting KRAS mutations found in non-small cell lung cancer and other cancers.
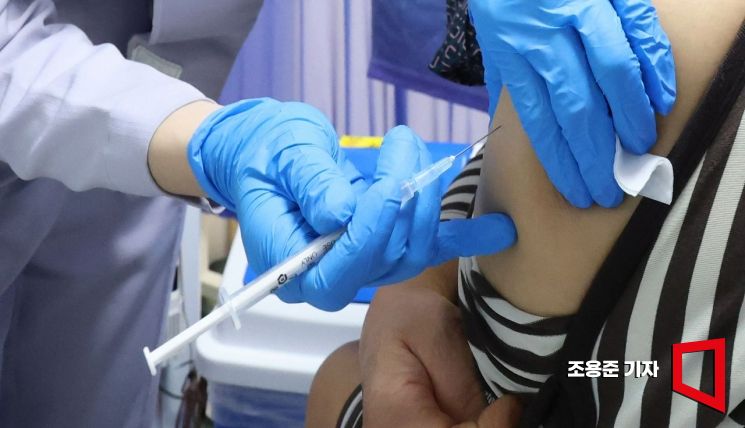 On the 19th, when free COVID-19 vaccinations began for high-risk groups and seniors aged 65 and older, an elderly person visiting a hospital in Gangseo-gu, Seoul, received the Pfizer vaccine. General individuals aged 12 to 64 who are not in the high-risk group will be able to receive free vaccinations starting from the 1st of next month. Photo by Jo Yongjun jun21@
On the 19th, when free COVID-19 vaccinations began for high-risk groups and seniors aged 65 and older, an elderly person visiting a hospital in Gangseo-gu, Seoul, received the Pfizer vaccine. General individuals aged 12 to 64 who are not in the high-risk group will be able to receive free vaccinations starting from the 1st of next month. Photo by Jo Yongjun jun21@
The most notable cancer vaccine overseas is Moderna’s mRNA-4157/V940, developed based on mRNA technology. It is undergoing Phase 3 clinical trials with MSD’s blockbuster immuno-oncology drug Keytruda in patients with melanoma, a type of skin cancer. In a prior Phase 2 trial, it reduced recurrence and mortality risk by 44% compared to Keytruda monotherapy. MSD showed high expectations by exercising a joint development and commercialization option for mRNA-4157 in 2021, paying Moderna $250 million (approximately 334 billion KRW). Additionally, development competition is intensifying with candidates such as Candel’s adenovirus-based pancreatic cancer vaccine CAN-2409 and Ultimovax’s mesothelioma vaccine UV1, both of which have received fast-track approval from the US FDA.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)














