IBS Nanoparticle Research Center Synthesizes World's Most Efficient Catalyst Producing 3.7L Hydrogen per Hour
Also Successfully Converts 98% of Waste Plastic into Hydrogen
Published in Nature Materials
A catalyst that converts waste PET bottles into eco-friendly hydrogen using sunlight has been developed with domestic technology.
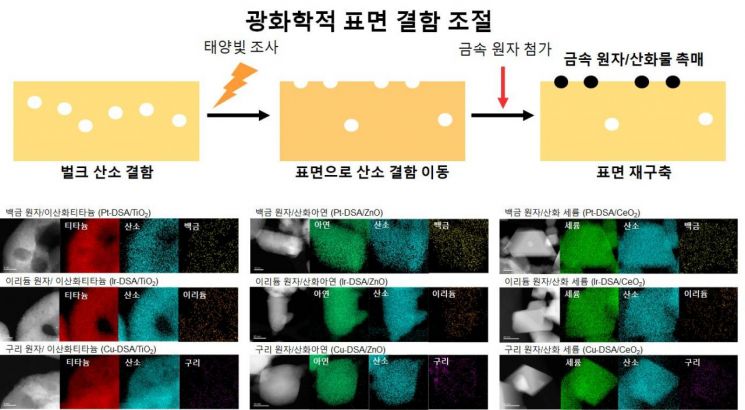
The Institute for Basic Science (IBS, President Noh Do Young) announced on the 6th that Hyun Taek Hwan, head of the Nanoparticle Research Division (Distinguished Professor, Department of Chemical and Biological Engineering, Seoul National University), together with Lee Byung Hoon, assistant professor at Korea University KU-KIST Graduate School of Converging Science and Technology (former IBS Nanoparticle Research Division researcher), and Professor Kim Min Ho's team at Kyung Hee University, jointly developed the world's most efficient catalyst that produces 3.7 liters of hydrogen per hour with just 1 gram.
 From the left, Hyun Taek-hwan, Director of the IBS Nanoparticle Research Division, Lee Byung-hoon, Assistant Professor at Korea University, and Lee Chan-woo, Researcher at the IBS Nanoparticle Research Division.
From the left, Hyun Taek-hwan, Director of the IBS Nanoparticle Research Division, Lee Byung-hoon, Assistant Professor at Korea University, and Lee Chan-woo, Researcher at the IBS Nanoparticle Research Division.
When this catalyst was applied to the photoreforming reaction of waste plastics, 98% of the plastic was converted into hydrogen. The research team evaluated the performance of a newly synthesized atomically dispersed platinum-titanium dioxide catalyst in a light-driven hydrogen generation reaction, showing the world's highest efficiency by producing 3.7 liters of hydrogen per hour using 1 gram of catalyst. When applied to the reaction producing hydrogen from plastics, it demonstrated the ability to convert 98% of waste plastics into hydrogen over 40 hours. This performance is more than 10 times higher than the previously reported best-performing catalysts.
Co-first author Lee Chan Woo, a researcher at the IBS Nanoparticle Research Division, explained, "This is a new synthesis method that can eco-friendly synthesize various high-performance atomically dispersed catalysts using infinite solar energy."
More than 5 billion PET bottles are consumed annually by the entire population of South Korea. However, due to the high recycling costs of waste PET bottles, the recycling rate does not reach 50%, and they are discharged as waste plastics causing environmental pollution. This development provides an opportunity to solve this problem.
This technology is evaluated to be more valuable as it presents a method to produce atomically dispersed catalysts, a core catalyst system that can significantly reduce chemical industry costs, in an eco-friendly and low-cost manner. Compared to existing synthesis processes, it enables catalyst production using only solar energy without any thermal energy. It also addresses the major drawback of existing nanoparticle-based catalyst systems that use expensive precious metals like platinum, which affects economic feasibility.
Professor Lee Byung Hoon of Korea University KU-KIST Graduate School said, "Since it is possible to create various high-performance atomically dispersed catalysts, we plan to apply catalysts synthesized by this method to various industrially important reactions."
Hyun Taek Hwan, the lead researcher, said, "Depending on the type of support and metal catalyst used, it can be synthesized diversely as photocatalysts or thermal catalysts, which can greatly reduce chemical industry costs," and added, "Since catalysts can be synthesized easily and quickly, it is expected to be easily scalable to industrial levels."
The research results were published online on February 6 at 1 a.m. (Korean time) in the international journal Nature Materials (IF 41.2).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.