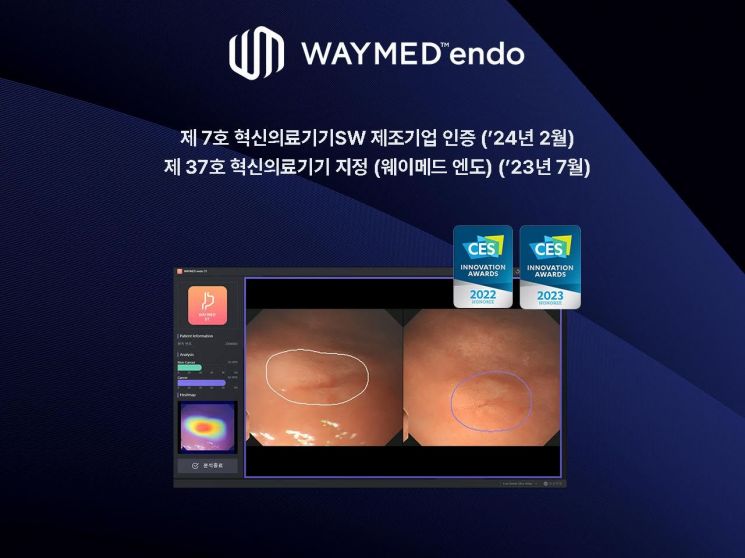
Weisen announced on the 5th that it has obtained certification as the '7th Innovative Medical Device Software Manufacturing Company' from the Ministry of Food and Drug Safety.
Weisen explained, "We received certification as an innovative medical device software manufacturing company in recognition of our continuous quality management efforts from product development to verification and maintenance, as well as the excellence of our risk management processes such as software issue resolution."
The Innovative Medical Device Software Manufacturing Company certification system evaluates the safety management level of medical device software manufacturers, certifies excellent manufacturers, and supports rapid product commercialization by exempting some documents during approval applications. Certified companies are exempt from submitting ▲comparison data with previously approved products ▲data on intended use ▲data on mechanism of action when applying for medical device product approval. Currently, companies designated as innovative medical device software manufacturers include Lunit, Vuno, and Coreline Soft.
Weisen was also designated as the 37th innovative medical device by the Ministry of Food and Drug Safety last July for its AI-based stomach and colon endoscopy examination analysis software, 'Weimed Endo.' A Weisen official said, "Through this innovative medical device software manufacturing company certification, Weisen has been recognized for its technological capabilities as a developer of high-quality innovative medical AI software," adding, "We are expanding our position as an innovative medical AI company."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















