High-Efficiency and Stable Water Electrolysis Catalyst
Can Replace Expensive Platinum and Iridium
A technology has been developed to produce high-purity green hydrogen in an environmentally friendly and cost-effective manner.
This technology, which replaces expensive precious metal catalysts, is attracting attention for its potential impact on carbon neutrality.
UNIST (President Yong-Hoon Lee) announced on the 11th that Professor Jeong-Gi Ryu of the Department of Energy and Chemical Engineering formed a joint research team with Professor Dong-Hwa Seo of the Department of Materials Science and Engineering at KAIST (President Kwang Hyung Lee) and developed a ‘bifunctional water electrolysis’ catalyst for producing high-purity green hydrogen with high efficiency and stability.
The developed catalyst was found to be usable for long periods even in highly corrosive acidic environments. It is based on ruthenium, silicon, and tungsten (RuSiW), making it cheaper than existing platinum (Pt) or iridium (Ir) catalysts. It is also environmentally friendly, emitting less than one-quarter of the greenhouse gases.
Water electrolysis is a technology that produces hydrogen by electrolyzing water. Since hydrogen can be produced without carbon emissions during the process, it is considered a next-generation technology for a carbon-neutral society.
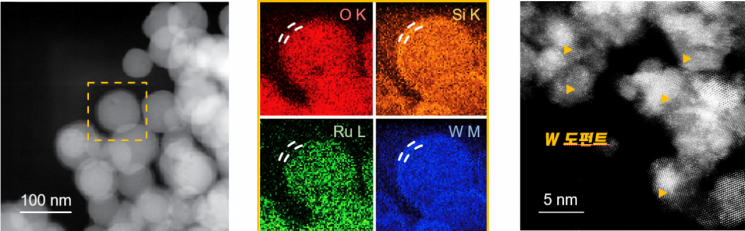 Transmission electron microscopy image of the developed RuSiW catalyst (left), elemental mapping image (center), and transmission electron microscopy image of tungsten-doped sample (right).
Transmission electron microscopy image of the developed RuSiW catalyst (left), elemental mapping image (center), and transmission electron microscopy image of tungsten-doped sample (right).
The research team studied materials that could replace precious metal electrolytes such as platinum or iridium, which are stable in acidic conditions. Ruthenium is recognized as an eco-friendly metal because its production cost is relatively low and it emits greenhouse gases at levels one-seventh and one-quarter lower than platinum and iridium, respectively.
However, ruthenium has lower catalytic activity than platinum and lower stability than iridium, which posed challenges for commercialization.
The research team developed a catalyst based on a ternary oxide of ruthenium, silicon, and tungsten. They simultaneously improved the functions of ruthenium catalysts, which have low stability in hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), demonstrating the potential of a bifunctional catalyst.
The developed catalyst has a structure where tungsten and silicon are doped around ruthenium atoms. It appropriately increases the adsorption strength of protons on the catalyst surface, enhancing the catalytic activity. It shows higher activity for the hydrogen evolution reaction than commercial platinum catalysts. A thin tungsten layer of about 5?10 nm protects the catalytic sites of ruthenium, improving stability.
The research team conducted catalyst stability tests. They passed a current of 10 mA through a 1 cm² electrode in an acidic electrolyte environment (pH 0.3). The developed catalyst operated stably for over 100 hours.
 Professor Jeongki Ryu of UNIST (from the left), first author researcher Hyunggu Kim, first author researcher Dasom Jeon (in the circle).
Professor Jeongki Ryu of UNIST (from the left), first author researcher Hyunggu Kim, first author researcher Dasom Jeon (in the circle).
Professor Jeong-Gi Ryu of the Department of Energy and Chemical Engineering said, “The developed ternary catalyst is significant because it can simultaneously replace expensive platinum and iridium. It is expected to be applicable to proton exchange membrane (PEM) electrolyzers, which are high-purity green hydrogen production systems, as it is stable for long periods even in highly corrosive acidic environments and can be easily synthesized.”
This research involved Dr. Dasom Jeon from UNIST’s Department of Energy and Chemical Engineering, Dr. Dong-Yeon Kim from KAIST’s Department of Materials Science and Engineering, and PhD candidate Hyun-Koo Kim from UNIST’s Department of Energy and Chemical Engineering as first authors.
The research was supported by the Ministry of Science and ICT, the National Research Foundation’s Mid-Career Researcher Support Program, the Regional Leading Research Center (RLRC) program, and the National Supercomputing Center (KISTI). The research results were published on January 4 in the world-renowned international materials science journal, Advanced Materials.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















