This year, the global pharmaceutical and biotech industry is recognized for being led by the issues of 'obesity drugs' and 'antibody-drug conjugates (ADC).' In South Korea, various pharmaceutical and biotech companies have also jumped into developing obesity drugs and ADCs.
◆Obesity Drugs Revitalize the National Economy=Obesity drugs developed by multinational pharmaceutical companies as glucagon-like peptide-1 (GLP-1) analogs have become global blockbusters. These compounds demonstrated over 20% weight loss effects, causing the developers' stock prices to soar. Many domestic pharmaceutical companies have also entered the race to develop GLP-1 obesity drugs.
Notable examples include Denmark's Novo Nordisk's 'Wegovy' (active ingredient semaglutide) and the United States' Eli Lilly's 'Mounjaro' (tirzepatide). Initially developed as treatments for type 2 diabetes by utilizing GLP-1's ability to promote insulin secretion and lower blood sugar, these drugs expanded into obesity treatment after GLP-1's appetite-suppressing effects were confirmed. GLP-1 is expected to expand its scope further to cardiovascular disease prevention, type 1 diabetes, liver diseases, and dementia treatments. Foreign media analyze that Novo Nordisk's rise to the top of the European stock market capitalization has even stimulated Denmark's economy, creating a national economic effect.
This trend is expected to continue next year. Mirae Asset Securities forecasted regarding the pharmaceutical and biotech outlook for next year, "Next year, obesity drugs will form a massive global pharmaceutical market and begin substantial growth." Mounjaro will be renamed 'Zepbound' and is scheduled for launch starting in the United States. Novo Nordisk is expanding its contract manufacturing organization (CMO) capacity, and Eli Lilly plans to operate a factory in North Carolina. As each company expands production capacity next year, the supply situation, which could not keep up with the surging demand throughout this year, is expected to improve.
The domestic pharmaceutical industry has also started to ride this wave this year. Numerous pharmaceutical companies have emerged aiming to develop obesity drugs. However, except for Hanmi Pharmaceutical's 'Epeglenatide,' which is recruiting patients for phase 3 clinical trials as a 'Korean-style obesity drug,' and Ildong Pharmaceutical's 'ID110521156,' which has received approval for phase 1 clinical trials, most are still in early development stages such as preclinical phases. There are concerns that if many pharmaceutical companies with insufficient financial resources and technological capabilities rush into obesity drug development, the situation seen during the COVID-19 period?where companies competitively announced vaccine and treatment development plans but ended up with no results?could repeat.
On the other hand, there is analysis that domestic technological levels are competitive. Jung Yuntaek, Director of the Pharmaceutical Industry Strategy Research Institute, said, "Although domestic pharmaceutical and biotech companies are clearly latecomers, the obesity drug market is not yet fully established, so catching up is possible." He added, "They can develop obesity drugs through joint development with global pharmaceutical companies, and by introducing new drugs that improve dosing convenience through formulation changes, unlike existing injectables, they can gain competitiveness." Ildong Pharmaceutical is developing ID110521156 as an oral drug, while Daewoong Pharmaceutical and Daewon Pharmaceutical are developing 'microneedle' obesity drugs in patch form, similar to a plaster.
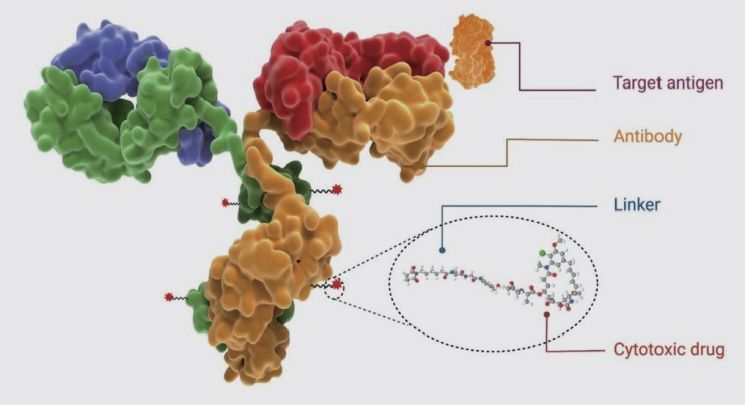
◆ADC Cancer Drugs, Global Technology Deals Continue=While obesity was the hot issue in terms of diseases for the pharmaceutical industry, ADC stood out as a technology platform. ADC is a technology that links an antibody that binds to cancer cells with a toxic drug (payload) that kills cancer cells into a single drug. It is called a 'cruise missile' for precise cancer targeting. AstraZeneca (AZ) and Daiichi Sankyo jointly developed the metastatic breast cancer treatment 'Enhertu' using ADC technology. When the median progression-free survival (mPFS) of patients without disease progression extended from 6 months with existing drugs to 28 months, other multinational pharmaceutical companies rushed to secure this technology.
In the global pharmaceutical market this year, the industry estimates that there have been 15 technology deals and mergers & acquisitions (M&A) related to ADCs exceeding $1 billion (approximately 1.3 trillion KRW). In March, Pfizer acquired Seagen, which owns three ADCs, for $43 billion (about 56 trillion KRW), and on the 26th, Korean company LegoChem Biosciences signed an export contract for the related technology 'LCB84' with Janssen for a total of $1.7225 billion (about 2.238 trillion KRW).
In the global market, this trend continues to accelerate, with major pharmaceutical companies like Pfizer and BMS acquiring ADC assets just this month. It is expected that next year, domestic ADC developers will also have opportunities to achieve results in the global market. Lee Seung-gyu, Vice Chairman of the Korea Bio Industry Association, said, "Opportunities for our pharmaceutical and biotech companies to export ADC technology will increase next year."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.