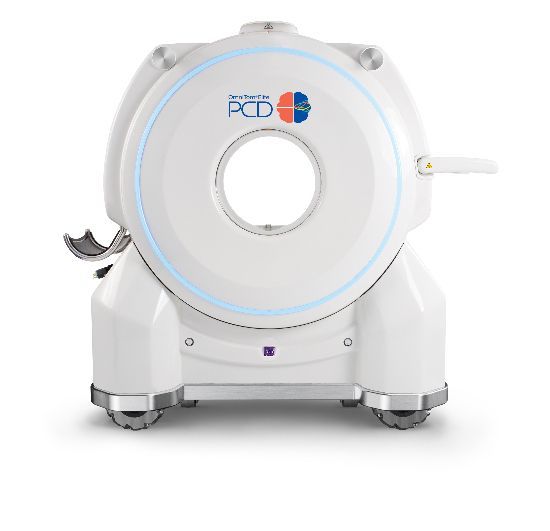
Introducing 'OmniTom Elite PCD' Approved by the US FDA
Samsung Electronics' Medical Device Business Division, Samsung Medison, and Neurologica announced on the 23rd that they will participate in the 'Radiological Society of North America (RSNA) 2023' held in Chicago, USA, from the 26th (local time) to the 30th, showcasing high-efficiency imaging diagnostic devices based on photon-counting detector (PCD) and artificial intelligence (AI) solutions.
Since 2012, Samsung has consistently introduced new technologies at RSNA, the world's largest conference in the field of medical imaging. This year, Samsung will unveil the 'OmniTom Elite PCD,' the world's first mobile CT equipped with PCD to receive approval from the U.S. Food and Drug Administration (FDA).
PCD is a next-generation CT technology utilizing semiconductors. Compared to conventional scintillator-based detectors, it provides CT images with higher resolution and lower noise. It separates and represents internal and external substances within the human body, enhancing diagnostic accuracy. Compared to existing methods, it also requires less contrast agent, making it suitable for patients who need to be cautious about kidney function.
Additionally, since it does not include electrical noise, it can reduce radiation exposure during imaging. All of Samsung's mobile CT devices have significantly reduced the amount of radiation emitted to the surroundings during imaging. This has been recognized, allowing their use not only in specialized imaging rooms but also in intensive care units and operating rooms.
Among Samsung's mobile CT device lineup, the 'OmniTom Elite' model features a wide bore, the space where the patient's body part is placed, enabling full-body imaging of pediatric patients under 7 years old in a single scan. The 'BodyTom 64' model, capable of full-body imaging for adults, was selected last month as the 'Best New Radiology Medical Device' by Antmini, a global radiology professional portal.
At this conference, Samsung will also exhibit its premium ultrasound diagnostic device lineup, including the 'RS85 Prestige' and 'V8.' Through an experience zone, participants will be able to directly explore AI-based diagnostic assistance features such as the real-time nerve tracking function 'NerveTrack.' High-performance battery-powered digital X-ray devices like the 'Ace GM85' will also be showcased.
Kim Yong-kwan, Head of Samsung Electronics' Medical Device Business Division and CEO of Samsung Medison, said, "Samsung is developing new products and technologies to advance medical technology and improve human health." He added, "We will continue to upgrade the next-generation mobile CT equipped with PCD technology to enhance convenience for medical professionals and patients."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.