KAIST Develops Electrode Material with 7 Times Greater Durability Compared to Existing Ones
Domestic researchers have developed a next-generation hydrogen fuel cell electrode material with a lifespan more than seven times longer than existing materials, signaling a green light for commercialization.
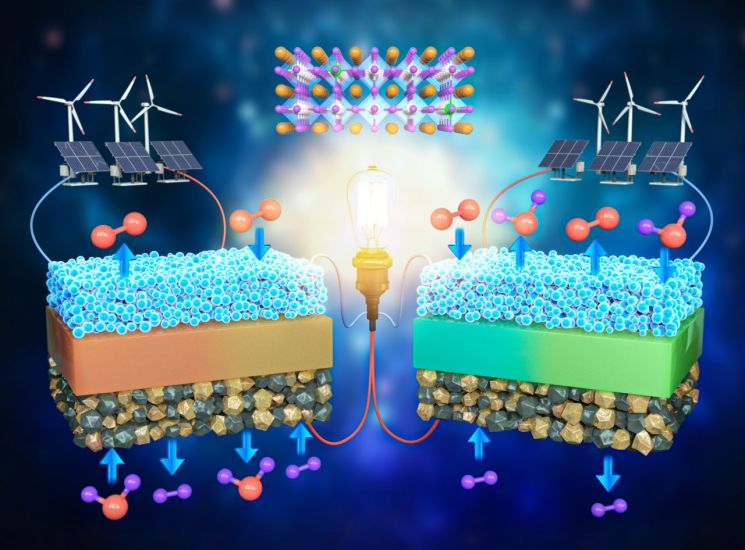
The Korea Advanced Institute of Science and Technology (KAIST) announced on the 9th that a research team led by Professors Woochul Jeong from the Department of Materials Science and Engineering and Kangtaek Lee from the Department of Mechanical Engineering, in collaboration with Professor Junhyuk Kim from Hongik University, successfully developed an electrode material applicable to both oxygen ion and proton-conducting solid oxide fuel cells.
Fuel cells are devices that produce electricity with high efficiency using hydrogen, a clean energy source. They are considered a key technology in the upcoming hydrogen society. Among them, ceramic fuel cells are divided into two types based on the type of ion transported through the electrolyte: oxygen ion-conducting solid oxide fuel cells (SOFC) and protonic ceramic fuel cells (PCFC). Furthermore, since both types can reversibly convert between electricity and hydrogen, they can be classified into four types of devices in total. These devices are emerging as next-generation core technologies for a carbon-neutral society, applicable to hydrogen electric vehicles, hydrogen refueling stations, and power generation systems.
However, these devices have a chronic problem where the efficiency significantly drops as the operating temperature decreases, due to the slowing of the slowest electrode reaction. Various studies have been conducted to address this issue, but most reported electrode materials suffer from low catalytic activity and are limited to specific devices. This posed limitations for application in solid oxide fuel cells that require reversible power conversion and hydrogen production.
To solve this problem, the research team succeeded in stabilizing a highly unstable crystal structure by doping a high-valence ion (Ta5+) into perovskite oxide materials, which had previously received little attention, and confirmed that the catalytic activity improved by more than 100 times.
The electrode material developed by the research team was applied to all four types of devices for power generation and hydrogen production in both oxygen ion-conducting solid oxide fuel cells (SOFC) and protonic ceramic fuel cells. Moreover, the efficiency of these devices was the highest reported to date, and unlike existing materials that degraded after 100 hours of operation, the developed electrode material demonstrated stable operation over a long period (700 hours), proving its excellence.
The research results were published online on the 12th of last month in the British Royal Society's journal in the field of materials and chemistry, Energy & Environmental Science (IF: 32.5). (Paper title: An Universal Oxygen-Electrode for Reversible Solid Oxide Electrochemical Cells at Reduced Temperatures)
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.