Neuin, an Electronic Medicine Developer
Beyond Treating Brain Disorders like Migraine and ADHD
Ambitions for Eye Disease Treatment through 'Regenerative Therapy'
and Cancer Treatment via 'Electric Field Stimulation'
"Nerves and other parts of our body regenerate themselves when injured or damaged. If we stimulate this process electrically to enhance regeneration effects, fundamental treatment for ophthalmic diseases with large unmet needs becomes possible."
 Kim Dohyung, CEO of Newein, is holding the migraine treatment device 'Ilexia' and explaining the product. [Photo by Newein]
Kim Dohyung, CEO of Newein, is holding the migraine treatment device 'Ilexia' and explaining the product. [Photo by Newein]
Electronic medicine is a therapeutic technology that provides smooth regeneration and function of nerves, tissues, and organs through electrical stimulation mimicking biological signals. Currently, most commercialized electronic medicines focus on treating brain-related diseases such as migraines and ADHD. However, NewAin is a company with ambitions to develop electronic medicines not only for brain diseases but also for a variety of other conditions.
Kim Dohyung, CEO of NewAin, said, "We aim to expand the scope of electronic medicine beyond the current level of common neuromodulation techniques such as transcranial direct current stimulation (tDCS), to regenerative treatments for ophthalmic diseases through regulation of cellular metabolic activities, and further to anticancer therapies."
For now, NewAin has successfully completed the development of brain disease treatment devices. The migraine treatment device 'Elexia' signed an exclusive domestic sales contract with Dong-A Pharmaceutical in June. Following a research and development (R&D) agreement last November, they have now partnered for sales as well. Domestic sales through Dong-A Pharmaceutical are expected to begin in October.
The ADHD treatment device 'Smile' received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and is scheduled to start clinical trials for upgrades next year. CEO Kim explained, "Although treatment devices have already been approved overseas, they are inconvenient for patients to use, so sales have been poor. We plan to improve usability and convenience as well as technology to compete effectively." The autism spectrum disorder (ASD) treatment device 'LittleBear' is also scheduled for release in 2025.
NewAin aims to launch ophthalmic disease treatment devices for the cornea and retina by next year. Given the high unmet needs, this was the initial target field at the company's founding. The company name 'NewAin' reflects this meaning. CEO Kim explained, "It combines the ancient word for light 'Nu' and the English word for eye 'eye,' symbolizing our goal to develop eye treatment products and bring the light of enlightenment to humanity."
The fastest developing ophthalmic device is 'Lux (NewAin01),' a dry eye treatment device through corneal regeneration. CEO Kim explained, "Nerves produce endogenous nutrients that regenerate themselves when injured, and the treatment protocol is to provide appropriate stimulation to increase this production." He added, "In exploratory clinical trials, we confirmed the treatment effect for dry eye syndrome. It helped regenerate corneal nerves damaged during surgeries such as LASIK, LASEK, and cataract surgery, alleviating dry eye and discomfort."
However, considering cost-effectiveness, the company plans to focus on dry eye cases primarily caused by direct corneal damage. CEO Kim said, "Lux shows significant effects in cases where the cornea is directly damaged by surgery or chemicals. While it also works for general dry eye syndrome, our device is currently expensive, so patient satisfaction may be lower compared to existing drugs for those cases."
The retinal treatment device 'LightSaver (NewAin02)' targets macular degeneration. Macular degeneration begins with dry macular degeneration caused by waste accumulation and hypoxia in the macula, the central part of the retina. If it progresses to wet macular degeneration with excessive blood vessel growth, it can lead to blindness. Wet macular degeneration is treated with direct eye injections, which only prevent symptom worsening but are not fundamental cures, and there is no treatment for dry macular degeneration.
CEO Kim said, "Our goal is to develop a fundamental cure. Since aging is the root cause, we aim to improve this." The basic mechanism is similar to that of the cornea, promoting regeneration. He added, "For wet macular degeneration, our goal is to enable dual treatment that suppresses neovascularization while promoting regeneration through combination clinical trials with existing injection drugs."
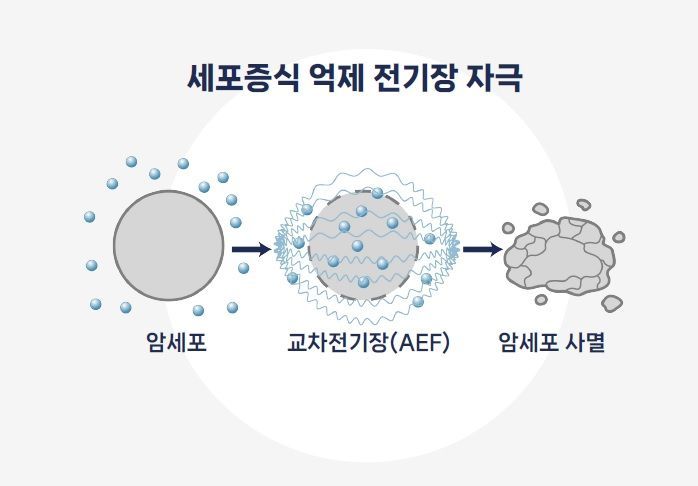 Schematic diagram explaining the anticancer adjuvant therapy mechanism of Newine through inhibition of cellular proliferation [Photo by Newine]
Schematic diagram explaining the anticancer adjuvant therapy mechanism of Newine through inhibition of cellular proliferation [Photo by Newine]
In the field of electronic medicine, anticancer treatment, which is even less familiar than regenerative therapy, works by "applying a strong electric field to disrupt cancer cell division," and "although about 10% of normal cells also die, the response is tumor-specific, allowing 80-90% of cancer cells to be eliminated," CEO Kim explained. Currently, this is at the animal testing stage. This technology is intended for combination therapy rather than as a primary treatment. CEO Kim said, "We want patients to be able to continue anticancer treatment at home. Since continuous management is required, if commercialized, it could generate steady revenue."
CEO Kim emphasized that not only the technology to deliver electricity but also the materials that can effectively transmit it are important, and the company is focusing on related research. He said, "The method involves attaching a ceramic device as a patch to the body to deliver an electric field inside. The key is ensuring the electric field reaches the cancer cells well, so we have been conducting material research to develop ceramics with ultra-high dielectric constants (degree of charge mobility)."
CEO Kim also presented the goal of steadily increasing sales and achieving a public listing soon. He said, "We expect to reach the break-even point around next year based on current standards, but since R&D investment must continue to increase, we will accept deficits for the time being." He added, "Having completed investment through Series C, we will begin full-scale listing preparations such as technology evaluations starting next year, aiming for a listing as early as the end of next year."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

















