UNIST, Clue to Developing Next-Generation Wearable Secondary Batteries
A method has been developed to charge secondary batteries using low thermal energy such as body temperature or heat energy generated from temperature differences between day and night. It is expected to bring us one step closer to the development of next-generation wearable secondary batteries.
The research team led by Professors Hyunwook Lee and Donghwa Seo from the Department of Energy and Chemical Engineering at Ulsan National Institute of Science and Technology (UNIST), in collaboration with Professor Seokwoo Lee’s team from Nanyang Technological University in Singapore, announced on the 27th that they identified key factors to improve the energy conversion efficiency of the thermally regenerative electrochemical cycle (TREC) system. They also developed a system capable of charging secondary batteries using heat energy generated from small temperature differences below 100°C.
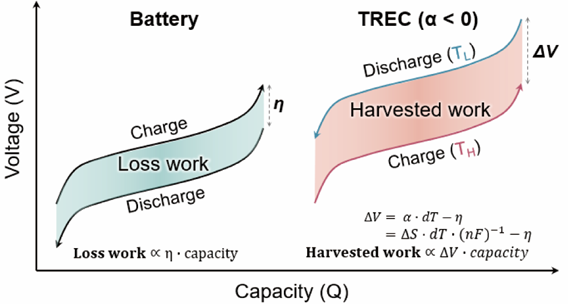
TREC (Thermally Regenerative Electrochemical Cycle) is an energy conversion system currently widely used and structurally identical to secondary batteries. It utilizes the voltage magnitude that varies with temperature to construct secondary batteries with electrode materials having different temperature coefficients. Heat energy generated from external temperature changes is converted into electrochemical energy inside the secondary battery. By using TREC, small amounts of energy can be charged from body temperature or temperature differences between day and night.
The research team studied methods to improve energy conversion efficiency to utilize TREC in various ways. They analyzed how the material structure inside the cathode affects performance and confirmed the results through computer simulations. Among TREC systems with electrolytes of the same composition, they designed the most efficient system and confirmed its successful operation under various temperature conditions. They found that the fewer water molecules the material contains, the greater the symmetry of the cathode material structure, which strengthens the bonding between transition metals and ligands near water molecules. This structural change activates the A1g vibrational mode with large vibrational energy, thereby increasing the structural vibrational entropy. Since entropy changes are closely related to the magnitude of the temperature coefficient, this ultimately improved the efficiency of the TREC system.
The research team explained, "We discovered that the intrinsic properties of the materials used in TREC significantly affect the energy conversion efficiency of TREC," adding, "This study provides meaningful new directions for future TREC research." Professor Lee also stated, "With the advancement of the Internet of Things, the development of wearable secondary batteries has become an issue, but there are limitations to charging with conventional conductors. For the development of body-attached batteries, new charging methods like the TREC system need to be discovered, and further development and research of next-generation batteries suitable for new applications are necessary."
The research results were published online on June 3 in the international journal in the energy and materials field, Advanced Materials.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















