IBS Achieves Success in Photocatalytic Synthesis of Nitrogen Heterocyclic Compounds
A new process technology has been developed that can produce key pharmaceutical raw materials in an eco-friendly way by minimizing environmental pollution and energy use through the use of light.
The Institute for Basic Science (IBS) announced on the 10th that the research team led by Seungwoo Hong, Deputy Director of the Molecular Activation Catalysis Research Division and a professor in the Department of Chemistry at KAIST, proposed a new chemical reaction synthesizing nitrogen heterocycles using photocatalysts and succeeded in introducing both ‘lactam’ and ‘pyridine,’ the main skeletons of pharmaceuticals, into a single molecule.
‘Nitrogen heterocycles’ are major components of medicinal compounds. They have a structure in which nitrogen atoms are inserted between carbon atoms bonded in a ring (cyclic) form, and drugs are synthesized by attaching functional groups to this structure. More than 60% of drugs approved by the U.S. Food and Drug Administration (FDA) contain nitrogen heterocyclic structures. Developing strategies that can easily synthesize nitrogen heterocycles is as important as discovering new drug candidates.
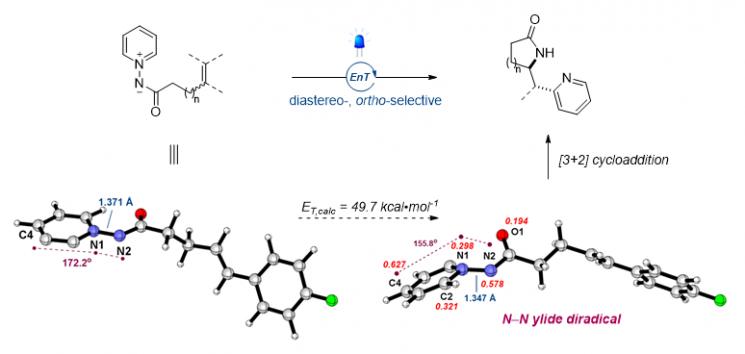
The research team newly proposed a strategy to synthesize useful substances by converting stable organic molecules into an unstable triplet state. First, the team computationally predicted that pyridinium salts, which are pyridine molecules attached with an amide group, can possess triplet energy. The triplet state is a molecular state where spins exist in one direction, which is very unstable and rarely found in nature. If the triplet state can be realized at room temperature, it can be applied to new chemical reactions that did not exist before. Subsequently, through actual experiments, the team generated pyridinium salts in the triplet state by utilizing photocatalysts that enable the pyridinium salts to absorb light energy and reach the triplet state.
Woo Seok Lee, the first author of the study, explained, “Through computational predictions and experimental verification, we reported a new chemical reaction called ‘triplet energy transfer.’ Unlike conventional synthesis methods that require adding reagents causing environmental pollution, this method is eco-friendly because it uses visible light.”
Furthermore, the team demonstrated for the first time that pyridine and lactam can be selectively generated simultaneously in a single molecule. Previously, introducing pyridine and lactam simultaneously required separate materials and multiple steps of chemical reactions, but now compounds with two functional groups selectively bonded can be synthesized in one reaction. This allows for more economical synthesis by combining major bioactive skeletons in one molecule and can also enhance drug efficacy. They also confirmed that the triplet energy transfer mechanism can be applied not only to pyridine but also to the synthesis of various ring-structured compounds.
Deputy Director Hong, who led the research, said, “Using triplet energy transfer can reduce the steps required for pharmaceutical synthesis. The process is not only simple but also eco-friendly, and it is expected to greatly benefit the entire industry, including the development of new drugs and various chemical products in the future.”
The research results were published online on May 27 (Korean time) in the prestigious chemistry journal Nature Chemistry (IF 24.274).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















