Development Underway to Expand Obesity Treatment Drugs
Up to 22.5% Weight Loss in Phase 3 Clinical Trials
Initially Approved as Diabetes Treatment in the US
Obesity Treatment Drug Expected to Be Approved Early Next Year
Eli Lilly's diabetes treatment 'Mounjaro' (generic name: Tirzepatide) has landed in South Korea. Currently, development is underway to expand its use as an obesity treatment, and clinical trials have confirmed a weight loss effect of up to 22.5%, marking the first time a weight reduction exceeding 20% has been observed, raising expectations as a 'miraculous obesity treatment.' Domestically, it has been initially approved as a treatment for type 2 diabetes.
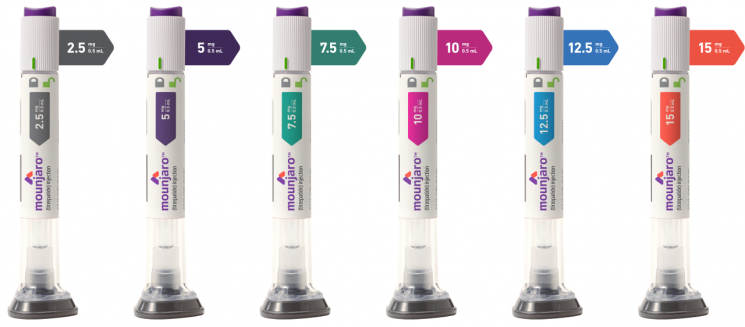
On the 28th, the Ministry of Food and Drug Safety announced that it had approved Mounjaro Prefilled Pen, an imported new drug from Korea Lilly, as an adjunct to diet and exercise therapy for blood glucose control in adult patients with type 2 diabetes. This approval covers six dosages: 2.5, 5, 7.5, 10, 12.5, and 15 mg/0.5 ml.
Mounjaro selectively binds to the insulin secretion-stimulating peptide (GIP) receptor and the glucagon-like peptide-1 (GLP-1) receptor, inducing blood glucose reduction before and after meals through mechanisms including ▲promotion of insulin secretion ▲improvement of insulin resistance ▲reduction of glucagon secretion. Through this, it has become the first synthetic peptide drug in South Korea with a mechanism that selectively binds to both GIP and GLP-1 receptors.
In May last year, Mounjaro was also approved by the U.S. Food and Drug Administration (FDA) as an adjunct to diet and exercise therapy for blood glucose control in patients with type 2 diabetes. While it has shown groundbreaking weight loss effects in phase 3 clinical trials, raising hopes as an obesity treatment, it has not yet been approved by the FDA for obesity treatment. However, since all clinical trials have been completed, development is underway aiming for FDA approval early next year.
The biggest competitor, Novo Nordisk's 'Wegovy' (semaglutide), was approved domestically in April. Since the diabetes treatment 'Ozempic,' which contains the same ingredient, was approved in April last year, Wegovy was recognized for the indication of weight loss as an adjunct to a reduced-calorie diet and increased physical activity for weight management, including weight loss and maintenance in adult patients. It is expected to be actually launched around the first half of next year.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















