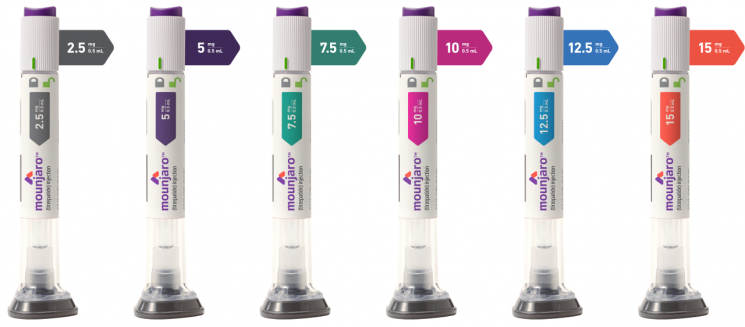
Lilly's 'Mounjaro' Approved as Diabetes Treatment
Obesity Drug Expected FDA Approval Next Year
American Diabetes Association, Frontline of Follow-up Drug Competition
Lilly, Boehringer Ingelheim, Novo Nordisk Lead the Race
Global big pharma companies are engaging in a fierce competition to quickly dominate the obesity treatment market, which is expected to grow to about 130 trillion won. Eli Lilly's 'Mounjaro' (active ingredient tirzepatide), which has garnered attention as a 'miracle obesity treatment' by demonstrating a weight loss effect of up to 22.5%?the first time exceeding 20% in clinical trials?has landed in Korea primarily as a diabetes treatment. Meanwhile, overseas, intense battles are underway to increase market share through higher weight loss efficacy and more convenient dosing methods.
According to the Ministry of Food and Drug Safety on the 3rd, domestic approval was granted on the 29th of last month for six dosages of Mounjaro prefilled pen. This approval was granted as an 'adjunct to diet and exercise for glycemic control in patients with type 2 diabetes.' In other words, approval was only given for weight loss and maintenance in diabetic patients, not for general obesity patients. Although clinical trials confirmed a 22.5% weight loss effect after 72 weeks of administering the highest dose of 15 mg/0.5 ml to obese patients without diabetes, there has been no approval yet for obesity treatment. Lilly plans to obtain FDA approval for obesity treatment within next year.
Overseas, various research and development (R&D) efforts are already underway to create follow-up drugs that surpass Mounjaro. The 'American Diabetes Association (ADA) 2023' conference held from the 23rd to 26th of last month at the San Diego Convention Center in California became a frontline of this competition, where big pharma companies such as Lilly, Boehringer Ingelheim, and Pfizer shared their latest research results.
This competition is driven by the remarkable growth of the market. Pharmaceutical market research firm IQVIA forecasts that the global obesity treatment market will grow to $10 billion (about 130 trillion won) by 2030. As Mounjaro's growth potential attracts attention, Lilly's market capitalization has grown to $440.9 billion (about 583 trillion won), surpassing Johnson & Johnson ($426.5 billion), which was previously the largest big pharma by market cap.
At this year's ADA, Lilly revealed a weight loss effect of 24.2%, surpassing Mounjaro's 22.5%, seemingly aiming to widen this gap further. The star is ‘Retatrutide,’ a triple agonist that simultaneously acts on glucagon-like peptide (GLP)-1, glucose-dependent insulinotropic polypeptide (GIP), and glucagon. In a phase 2 clinical trial involving 281 adults aged 18 to 75, the highest dose group of 12 mg showed a 24.2% weight loss effect after 48 weeks.
Recently, obesity treatment R&D has been dominated by development targeting GLP-1, GIP, and glucagon receptors. GLP-1 was originally developed as a diabetes treatment because it promotes insulin secretion and lowers blood sugar, but it has been repurposed as an obesity treatment after confirming secondary effects such as appetite suppression in the brain and delayed gastric emptying. GIP, although acting on different cell types than GLP-1, similarly promotes insulin secretion. Glucagon is secreted by the pancreas and promotes energy expenditure, leading to weight loss. For example, Mounjaro was developed as a dual agonist of GLP-1 and GIP, and Novo Nordisk’s 'Saxenda (liraglutide)' and 'Wegovy (semaglutide),' which currently dominate the obesity treatment market, are GLP-1 analogs. Various combinations are being developed to enhance efficacy.
In addition, Lilly disclosed phase 2 obesity clinical trial results showing that the GLP-1 agonist 'Orforglipron' reduced patient weight by up to 14.7%. Although its weight loss effect is lower than Mounjaro’s, it is an oral medication rather than an injection, which is expected to meet unmet needs for obese patients who have difficulty administering injections themselves.
Meanwhile, Novo Nordisk is also targeting unmet needs by releasing results from clinical trials converting Wegovy into an oral treatment. In late-stage clinical trials, it showed a 15% weight reduction effect in overweight and obese adults. Among 667 patients administered 50 mg of semaglutide, an average weight loss of 15.1% was confirmed after 68 weeks. Novo Nordisk plans to seek approval in the U.S. and Europe within this year. The same ingredient is already marketed as the oral diabetes treatment 'Rybelsus.'
Boehringer Ingelheim also revealed phase 2 clinical trial results for 'Serdutide,' a GLP-1 analog candidate jointly developed with Zealand Pharma. Among 373 overweight or obese patients, over 40% in the high-dose groups (3.6 mg, 4.8 mg) achieved at least 20% weight loss. The placebo group showed only a 2.8% weight loss. Wahid Jamal, Vice President of Boehringer Ingelheim, explained, "The 4.8 mg dose group had not yet reached the peak weight loss effect even after 46 weeks of clinical trials," adding, "Longer treatment could achieve additional weight loss."
However, GLP-1 agonists still carry significant concerns about side effects such as gastrointestinal disorders. Serdutide had a 29% dropout rate in the high-dose group during clinical trials, and although Retatrutide’s side effects were mild to moderate and alleviated over time, gastrointestinal issues were common. Orforglipron also caused nausea in 58% of patients in the mid-dose group.
Notably, Pfizer announced the discontinuation of a candidate drug due to failure to overcome side effects. In clinical trials of the oral obesity treatment 'Lotiglipron,' elevated liver enzymes were observed. Consequently, Pfizer’s stock price dropped sharply by 3.68% to close at $36.89 on the 26th of last month (local time). However, Pfizer is accelerating development of 'Danuglipron,' an oral drug taken twice daily. Unlike Lotiglipron, Danuglipron did not show elevated liver enzymes. Still, in phase 1 diabetes trials, Danuglipron raised concerns about increased alanine aminotransferase?a biomarker for liver disease?and potential heart rhythm issues.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.