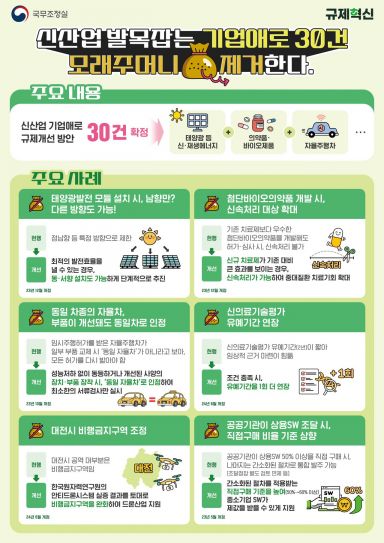
‘Regulatory Improvement Measures for New Industry Business Difficulties’
The government has decided to lift 30 regulations in new industry sectors such as renewable energy and pharmaceuticals/biotechnology. On the 15th, the New Industry Regulation Innovation Committee announced through the Regulatory Reform Committee that it had finalized the "Regulatory Improvement Plan for New Industry Corporate Difficulties."
This plan was prepared after the New Industry Regulation Innovation Committee, an advisory body to the Presidential Regulatory Reform Committee, identified the difficulties faced by companies in the field. Since January, the committee has discovered proposed tasks and, through 16 discussions with relevant ministries and companies, derived the plan.
According to the plan, the regulation on the "south-facing installation principle" for solar power generation modules will be eased. Although technology to increase power generation through east- and west-facing solar modules has advanced, domestic regulations required installation facing due south, making it difficult to apply new technologies. Going forward, installers will be allowed to independently determine efficiency based on the product and installation method to optimize power generation efficiency.
Additionally, to guarantee treatment opportunities for patients with serious diseases, the scope of expedited processing for excellent advanced biopharmaceuticals will be expanded. If a newly developed advanced biopharmaceutical for treating a serious disease shows significantly improved effects compared to existing treatments, it will be designated for expedited approval and review. This regulation revision aims to activate research and development and ensure treatment opportunities for patients.
Furthermore, the scope of recognition for the same autonomous vehicle eligible for simplified approval procedures when applying for temporary operation permits for autonomous vehicle development has been expanded, and regulations related to the extension of the grace period for new medical technology evaluation have been eased.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.