The research team led by Professor Chan-Young Park from the Department of Life Science at UNIST recently discovered a new regulatory mechanism of cell death in cancer cells, providing new clues to enhance the understanding of cancer development.
Normal cells undergo apoptosis under stress conditions such as nutrient deprivation and hypoxia. However, cancer cells overcome apoptosis even under stress and continuously proliferate, developing mechanisms favorable for their own survival.
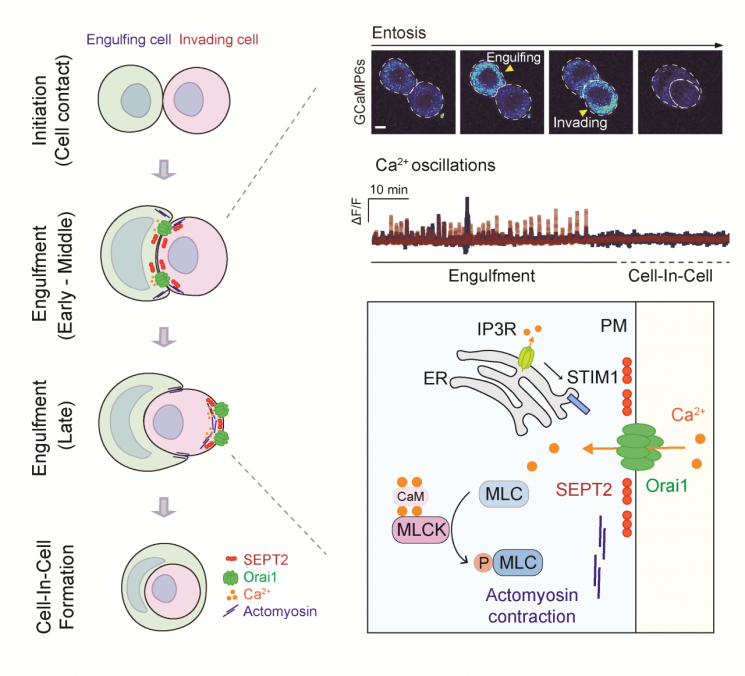
Recently, a form of non-apoptotic cell death called entosis has been reported. Entosis is a type of cell cannibalism phenomenon where a living cancer cell (invading cell) invades another cancer cell (host cell), forming a "cell-in-cell" structure.
Cancer cells create a favorable environment to evade cell death through entosis. The host cell receives nutrients and chromosomes from the invading cell, while the invading cell hides inside the host cell and may exit under appropriate conditions or even divide within the host cell.
This entosis influences cancer initiation and progression by causing genomic instability such as chromosomal abnormalities through interactions among cancer cells. Therefore, understanding entosis is essential for studying the causes of cancer and developing treatment methods.
According to the research team, cancer cells that are motile in the early stages of cancer development (invading and host cancer cells) trigger unique signaling mechanisms either within or between cells.
Among these, calcium channel-dependent protein signaling has been known as a very important mechanism for cancer cell signaling and interactions between cancer cells, but its association with entosis had not been studied.
In this study, the team revealed that the signaling mechanism of Orai1, a calcium channel protein located on the cell membrane, is essential for inducing entosis in cancer cells.
They observed that Orai1 locally translocates to specific regions of the cell membrane in both invading and host cancer cells, mediated by septin, a cytoskeletal protein, and exhibits characteristic oscillation patterns at the same location, causing partial concentration changes inside the cell.
This calcium signaling mechanism phosphorylates myosin, a motor protein essential for cell movement, inducing cytoskeletal rearrangement or cell motility.
As a result, the research team was able to elucidate the mechanism by which entosis is induced and progresses. They also found that regulating the Orai1 channel or its signaling pathway suppresses entosis, suggesting the possibility of controlling cancer development dependent on entosis.
Professor Chan-Young Park of the Department of Life Science stated, “This study elucidated a new signaling mechanism of the Orai calcium channel involved in the induction of entosis (cell-in-cell phenomenon) in cancer initiation and metastasis.” He added, “We expect that future research on regulating calcium channel signaling and entosis will contribute to cancer development, metastasis, and therapeutic strategy studies.”
This research was supported by the Ministry of Science and ICT-Korea Research Foundation’s Global Ph.D. Fellowship Program, Mid-Career Researcher Support Program, and Leading Research Center. The research results were published online on March 24 and in print on May 17 in the international journal Advanced Science.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















