Application for MFDS Approval in 2021... Introduction Failed Last Year
Japan Allows Use Up to 9 Weeks of Pregnancy... Strict Management
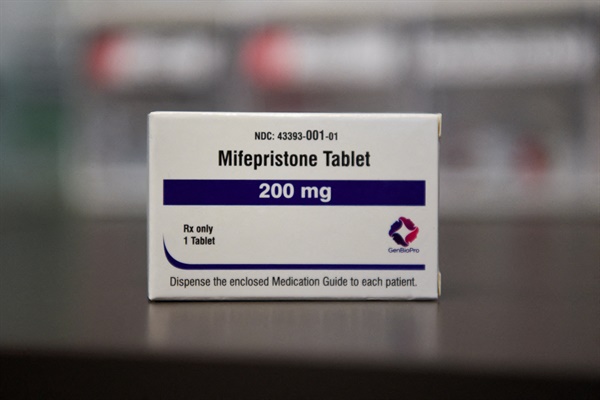
Last year, the introduction of oral pregnancy termination (abortion) pills was halted in Korea, but the same medication has been approved in Japan. The Japanese Ministry of Health, Labour and Welfare conditionally approved the manufacture and sale of the oral abortion pill ‘Mefigo Pack’ on the 21st of last month after several rounds of review delays.
Previously, there had been efforts to introduce the same drug in Korea, but they ultimately failed. After the 2019 Constitutional Court ruling that declared the abortion law unconstitutional, Hyundai Pharm, which had an exclusive contract with Line Pharma in Korea, applied for approval of the same drug under the name ‘Mifujimiso’ to the Ministry of Food and Drug Safety in 2021. However, as the process proceeded slowly, the application was voluntarily withdrawn at the end of last year.
On the 29th of last month (local time), the UK’s Guardian reported that the Japanese government had approved an abortion pill for the first time and briefly introduced the process leading up to the approval.
The Guardian mentioned that there is controversy surrounding mifepristone, the only oral abortion pill effectively available in the United States. Previously, a federal court in Texas ruled to revoke the FDA’s approval of the oral abortion pill (mifepristone). Subsequently, the U.S. Supreme Court extended a temporary stay on the enforcement of that ruling.
In Japan, the drug can be prescribed to women up to 9 weeks of pregnancy. The method involves first taking ‘mifepristone,’ which suppresses the action of the corpus luteum hormone necessary for pregnancy maintenance, followed by taking ‘misoprostol,’ a uterine contraction agent, 36 to 49 hours later.
Until an appropriate medical system is established, patients are instructed to wait at the hospital after taking the second drug until pregnancy termination is confirmed. To prevent misuse and abuse, pharmaceutical companies and medical institutions are also required to report monthly sales and usage volumes to the medical associations of the regional governments.
Japan conducted a strict approval process. Although Mefigo Pack has been designated as an essential medicine by the World Health Organization (WHO) for over 20 years and has been used in more than 80 countries for a long time, it underwent separate clinical trials within Japan.
In clinical trials involving a total of 120 participants, 90% confirmed pregnancy termination within 8 hours, and 93% within 24 hours. Some experienced side effects such as lower abdominal pain and vomiting, but most were mild, and there were four reported cases of abnormal bleeding or bacterial infection.
Within Japan, there is a generally positive atmosphere regarding the approval of abortion pills, emphasizing the need to guarantee women’s right to self-determination. The Asahi Shimbun reported that although there is significant opposition to abortion itself, many people supported the approval of abortion pills on the condition that they are strictly regulated.
Professor Tsukahara Kumi of Kanazawa Graduate School, who has studied abortion issues for the past 20 years, stated, “The rights to contraception and abortion are essential to eliminating discrimination against women and are important issues for all women,” adding, “A policy system that provides safe contraception and abortion must be established.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.