Professor Oh Jeong-su's Research Team at Sungkyunkwan University
Confirms Involvement of MDC1 and TOPBP1 Proteins
Domestic researchers have identified a new mechanism by which oocytes repair damaged DNA, laying an academic foundation to prevent the decline in oocyte quality caused by DNA damage and to increase the success rate of assisted reproductive technologies.
The Korea Research Foundation announced on the 20th that Professor Oh Jeong-su's research team at Sungkyunkwan University has elucidated a DNA damage repair mechanism that occurs specifically in oocytes.
Women are born with all the oocytes they will use throughout their lifetime stored in their ovaries. Because oocytes remain arrested at the early stage of meiosis in the ovaries for a long time, they are more vulnerable to DNA damage compared to somatic cells. Additionally, during the in vitro culture process in assisted reproductive technologies, increased reactive oxygen species can induce DNA damage. Oocytes with damaged DNA experience impaired embryo development and have higher risks of infertility, miscarriage, and birth defects.
All cells in the human body possess mechanisms to detect and repair damaged DNA to maintain genetic stability. The research team had previously confirmed that oocytes also have mechanisms to detect and repair DNA damage. Building on this, the team succeeded in specifically identifying the DNA damage repair mechanism in oocytes.
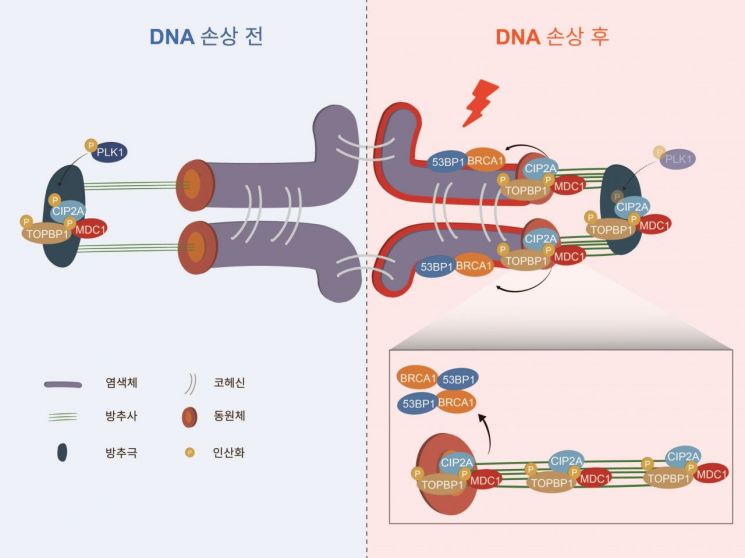
First, they observed the movement of MDC1 and TOPBP1 proteins involved in DNA damage repair during the detailed maturation process of oocytes. They confirmed that these factors, which normally gather at the spindle poles of oocytes, relocate to the chromosomes after DNA damage occurs. This revealed a new DNA damage repair mechanism unique to oocytes.
The research team found that when DNA damage occurs during oocyte maturation, chromosomes and spindle fibers interact to transport DNA repair factors from the spindle poles to the chromosomes via a pathway connecting the spindle pole to the spindle-kinetochore. They also identified that the movement of DNA repair factors is mediated by the CIP2A protein and regulated through the PLK1 kinase.
Professor Oh stated, “This study provides fundamental information necessary for developing strategies to preserve female fertility. Moving forward, we plan to conduct research to control the DNA damage repair capacity of oocytes to prevent oocyte aging and quality decline and to develop therapeutic methods.”
The results of this study were published on the 31st of last month in the international journal in the field of nucleic acid research, Nucleic Acids Research.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.