Welt 'PillowRx' Approved by MFDS
Insomnia CBT Treatment DTx
The growth of the domestic DTx industry is expected to accelerate as the second digital therapeutic device (DTx, digital therapeutic) has been released just two months after the first one.
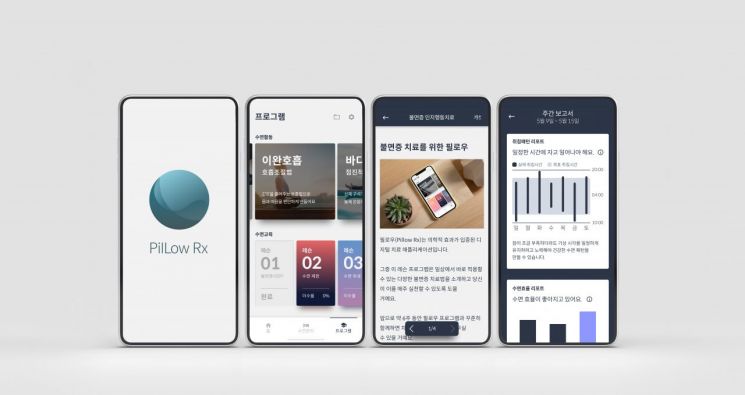
On the 19th, the Ministry of Food and Drug Safety announced, "We have approved the second domestic DTx, a cognitive therapy software developed by Welt, which applied for manufacturing approval." Named PillowRx (Welt-I), the second DTx is a software medical device (SaMD) that implements cognitive behavioral therapy (CBT) for insomnia through a mobile application (app). Based on the 'sleep diary' data entered by the patient, it provides personalized recommended bedtime, intervenes in patient behavior to improve sleep quality, and performs these interventions over six weeks to improve the patient's insomnia.
The Ministry of Food and Drug Safety explained, "We held a 'Medical Device Committee' composed of mental health experts from the Korean Neuropsychiatric Association and the Korean Digital Therapeutics Association to provide advice on safety and efficacy, and approved it after a scientific and thorough review." It added that since the first DTx, Aimmed's 'Somzz,' has a similar indication, "there is a commonality in having the same product composition and intended use," but "since the manufacturers are different, the algorithms differ, resulting in differences in user interface (UI), user experience (UX), and usage period." Somzz's usage period is 6 to 9 weeks, while PillowRx's usage period is 6 to 8 weeks.
Handok will handle the future sales of PillowRx. Handok and Welt have maintained cooperation since signing a share investment and partnership in 2021, with Handok holding the domestic distribution rights for PillowRx. Since Handok distributes 'Stillnox,' the most prescribed sleeping pill currently, synergy is expected in the insomnia field.
According to the Ministry of Food and Drug Safety's definition, DTx is "SaMD that provides evidence-based therapeutic interventions to patients to prevent, manage, or treat medical disorders or diseases." It can be installed and used on general-purpose devices such as smartphones and tablets without special equipment, and is used not only for treatment but also for prevention and management of diseases classified in actual disease classifications. It is a medical device that has established evidence of its mechanism of action through clinical trials. In particular, it greatly enhances patient convenience by allowing treatment anytime and anywhere, and has recently attracted attention because it can be developed faster than conventional new drugs.
With Active Government Support... Can the DTx Industry Take Root?
The rapid release of the second DTx following the first is largely credited to the efforts of related authorities, including the Ministry of Food and Drug Safety. The domestic DTx regulatory level is recognized by overseas experts as world-class and a model case.
The Ministry of Food and Drug Safety issued the world's first 'DTx Approval and Review Guidelines' in August 2020 and continues to implement tailored regulations by creating 'DTx Safety and Performance Evaluation and Clinical Trial Protocol Preparation Guidelines' for various indications including insomnia.
 The Ministry of Food and Drug Safety established the world's first "Digital Therapeutics (DTx) Approval and Review Guidelines" in August 2020. [Provided by the Ministry of Food and Drug Safety]
The Ministry of Food and Drug Safety established the world's first "Digital Therapeutics (DTx) Approval and Review Guidelines" in August 2020. [Provided by the Ministry of Food and Drug Safety]
During the approval process of PillowRx, the Ministry designated PillowRx as an approval assistant target in July 2021 and provided specialized support such as insomnia DTx guidelines. Additionally, in December last year, through the 'Innovative Medical Device Integrated Review and Evaluation System' prepared with related ministries, PillowRx was selected as the first innovative medical device, achieving a reduction of about 80% in the time from approval to use in medical settings.
Kang Sung-ji, CEO of Welt, said, "The Ministry of Food and Drug Safety's prompt regulatory establishment and tailored consultations were the driving force that enabled the rapid launch of DTx," adding, "We will further develop PillowRx by exporting it worldwide."
Oh Yu-kyung, Commissioner of the Ministry of Food and Drug Safety, also stated, "We will make domestic regulations a global standard so that domestic companies can continue to develop innovative products leading the global market," and added, "We will strengthen regulatory support bridges such as close consultations with regulatory experts and application of global standards to increase the success rate of product development and accelerate product launches."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.