SK Biopharm announced on the 29th that it has received approval from the Ministry of Food and Drug Safety for the Phase 3 clinical trial plan (IND) of the innovative epilepsy drug 'Cenobamate' for adolescent generalized seizure epilepsy.
This Phase 3 clinical trial is part of a multinational study to evaluate the efficacy and safety of Cenobamate in patients aged 12 to under 18 years with primary generalized tonic-clonic seizures.
The domestic Phase 3 trial will be conducted at five clinical institutions, including Seoul National University Hospital, involving about 30 adolescent patients. It will be a randomized, placebo-controlled, double-blind study. Enrolled patients will receive Cenobamate or placebo for approximately 34 to 37 weeks. Afterwards, patients may continue treatment for up to one year in an open-label phase, depending on their choice.
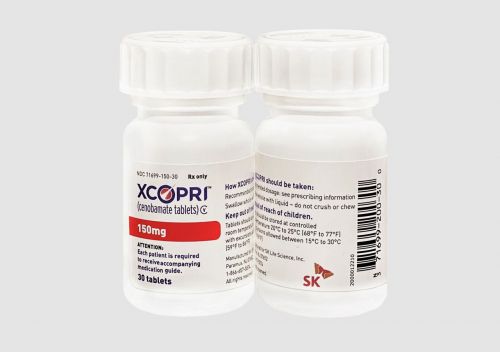
Cenobamate is an epilepsy drug that SK Biopharm independently developed from discovery to approval, showing a significant seizure-free rate in adult epilepsy patients. It was approved by the U.S. Food and Drug Administration (FDA) in November 2019 for partial-onset seizures in adults and received marketing authorization from the European Commission (EC) in March 2021, and is currently on the market.
To expand the indications to include generalized tonic-clonic seizures and to extend the eligible age range from adults to adolescents, multinational clinical trials are being conducted in eight countries including South Korea, the United States, Australia, and Germany. Additionally, a Phase 3 clinical trial for adult patients with partial-onset seizures is ongoing domestically.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















