Celltrion Healthcare's antibody biosimilar 'Remsima' (generic name infliximab) has surpassed a global cumulative prescription amount of 12 trillion KRW and cumulative sales of 5 trillion KRW.
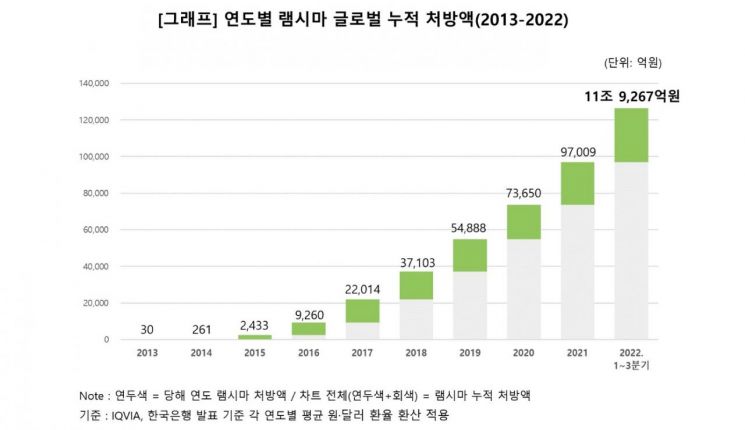
Celltrion Healthcare announced on the 21st that according to the pharmaceutical market research firm IQVIA, Remsima recorded a global cumulative prescription amount of 11.9267 trillion KRW from 2013 to the third quarter of 2022, spanning about 10 years. The prescription amount exceeded 12 trillion KRW as of the end of last year.
Furthermore, the company explained that from 2013, when Remsima sales first occurred at Celltrion Healthcare, until last year, the global cumulative sales reached 5.1631 trillion KRW over 10 years. Among pharmaceutical products developed and sold by domestic pharmaceutical and bio companies, Remsima is the first single product to exceed a global cumulative prescription amount of 12 trillion KRW and cumulative sales of 5 trillion KRW.
Remsima recorded a 52% market share in Europe in the fourth quarter of 2017, becoming the first antibody biosimilar to surpass the market share of the original drug. In 2017, it set a record as the first domestic pharmaceutical product to exceed an annual prescription amount of 1 trillion KRW, with over 1.2 trillion KRW prescribed globally in one year.
The company stated that Remsima's prescription performance continues to this day. According to IQVIA, as of the third quarter of last year, Remsima held a 55% market share in Europe. In particular, it showed an 82% market share in the United Kingdom and 66% in Spain. Thus, Remsima has maintained the number one position for infliximab for six consecutive years since 2017. The company explained that the results were achieved by leading bidding competitions with customized strategies considering market characteristics in each country through local subsidiaries established in 15 European countries.
In the United States, Remsima recorded a 32% market share in January and has maintained the number one position for infliximab biosimilar prescriptions since its launch. In Brazil, the largest pharmaceutical market in Latin America, it has secured over 80% market share by winning federal and state government bids for two consecutive years, and in Japan, it maintains the number one position for biosimilar prescriptions with a 26% market share.
Celltrion Healthcare forecasts that the expansion of Remsima prescriptions will continue. This is because Remsima is supplied globally across North America, Europe, Asia, and Latin America, and market expansion continues with recent sales approvals in new regions.
A Celltrion Healthcare official said, "The significance of the global cumulative prescription amount of 12 trillion KRW is that Remsima has faithfully fulfilled its role as a gift-like treatment enabling patients suffering from autoimmune diseases worldwide to return to their daily lives for a long time," adding, "We will do our best to continue surpassing a cumulative prescription amount of 20 trillion KRW as Remsima leaves another new milestone in the domestic bio-pharmaceutical industry."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















