IBS Joint Research Team
A path has been opened to environmentally friendly mass production of hydrogen peroxide, a key chemical in the chemical industry, using only oxygen and water. Costs and carbon emissions are also drastically reduced, increasing the potential for commercial use through further research.
The Institute for Basic Science (IBS) announced on the 14th that a joint research team including Hyun Taek-hwan, director of the Nanoparticle Research Division (Distinguished Professor, Department of Chemical and Biological Engineering, Seoul National University), Sung Young-eun, deputy director (Professor, Department of Chemical and Biological Engineering, Seoul National University), and Edward Saljent, professor at the University of Toronto, Canada, developed a new electrocatalyst capable of producing hydrogen peroxide with the world's highest efficiency. The catalyst can produce 6.6 tons of hydrogen peroxide per day with just 1 kg of catalyst, surpassing the team's own 2020 record of 341.2 kg.
 Schematic Diagram of the Catalyst Developed by the Research Team
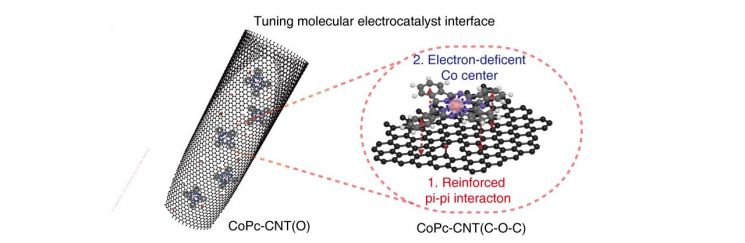
Schematic Diagram of the Catalyst Developed by the Research TeamThe research team developed a new electrocatalyst that stabilizes CoPc (Cobalt Phthalocyanine) with a planar structure on carbon nanotubes. Using this catalyst, they were able to electrically produce a high concentration of hydrogen peroxide at 5wt%. While using only eco-friendly electrical energy, the energy consumed for hydrogen peroxide production was only about one-fourth of that in the industrial anthraquinone process (16 GJ/ton vs 70 GJ/ton).
Hydrogen peroxide is widely used not only in everyday products such as toothpaste and kitchen detergents but also in medical settings requiring sterilization and semiconductor processes that require impurity removal. Currently, hydrogen peroxide is produced by the anthraquinone process, which involves adding hydrogen to an organic compound called anthraquinone and then oxidizing it with air. However, this process requires large amounts of expensive precious metal catalysts such as palladium, is complex, consumes a lot of energy, and produces organic byproducts that cause environmental pollution.
To overcome these drawbacks, recently, an electrochemical method that produces hydrogen peroxide (H2O2) by adding electrons to oxygen (O2) through a reduction process has attracted attention. This method does not require high pressure or high temperature and produces no byproducts, making it clean. However, a suitable industrial catalyst for this process has not yet been developed.
In 2020, the research team developed a cobalt-based catalyst, a cheap metal instead of precious metals, and demonstrated for the first time that hydrogen peroxide can be produced electrochemically using only water and oxygen. Cobalt costs about 30,000 KRW per kilogram, which is much cheaper than palladium (about 61 million KRW) or platinum (about 40 million KRW), which have been used as catalysts. Although this catalyst showed world-class hydrogen peroxide production efficiency at the time, it was only active at a laboratory scale more than 100 times smaller than actual industrial scale. In this study, the catalyst was improved to maintain the previous advantages while showing high activity at an industrial scale.
Co-author Byung-Hoon Lee, a postdoctoral researcher at the University of Toronto, Canada, explained, “The catalyst developed in 2020 was cobalt atoms placed on graphene, but in this study, a molecular catalyst was combined with carbon nanotubes (CNT), a cylindrical carbon material. Molecular catalysts show higher performance than catalysts mainly used in industry but have limited use due to lower stability, and we solved that problem.”
The new catalyst showed high hydrogen peroxide production efficiency even at industrially applicable current densities (above 0.3 A/cm²) and can produce high-concentration hydrogen peroxide (5 wt%) used in daily life. It also secured stability by maintaining more than 99% of its initial performance even after continuously producing hydrogen peroxide for over 100 hours. The energy consumed to produce 1 ton of hydrogen peroxide is 16 GJ (gigajoules), only about one-quarter of the existing anthraquinone process (70 GJ/ton).
Deputy Director Sung Young-eun said, “We plan to improve the catalyst to electrically produce ultra-high concentration hydrogen peroxide (30 wt%). Hydrogen peroxide is diluted with water to the desired concentration, but producing highly concentrated hydrogen peroxide can reduce transportation costs, resulting in greater economic benefits.”
Director Hyun Taek-hwan said, “This will have a significant impact on academia as it can lead not only to hydrogen peroxide but also to the electrochemical production of various useful chemical fuels using molecular catalysts.”
The research results were published online on the same day in the international journal Nature Catalysis (IF 40.71).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















