Seoul National University Research Team Uncovers Mechanism Inducing Cholesterol Aging
High cholesterol levels in the body adversely affect health through various cardiovascular diseases, including heart disease. Numerous epidemiological studies have well established that abnormalities in cholesterol are also observed in aging and various aging-related diseases, but the causal relationship has remained a mystery. Recently, a domestic research team newly uncovered the mechanism by which cholesterol induces aging, providing an important clue to solving this challenge.
Seoul National University announced on the 7th that the research team led by Professors Changhee Kang and Jinhong Kim from the Department of Biotechnology identified the regulatory and functional mechanisms of cholesterol that cause cellular senescence and aging-related inflammatory responses.
Cellular senescence is a phenomenon in which normal cells permanently stop dividing due to stress and secrete various inflammatory factors. Recently, the accumulation of senescent cells has been recognized as a major cause of several aging-related diseases such as cancer, cardiovascular diseases, and degenerative diseases, and senotherapy, a technology targeting senescent cells, is receiving global attention as a next-generation anti-aging therapeutic candidate.
 Aging image.
Aging image.
Representative examples include the launch of the large-scale research project “Senescence Network (SenNet, $125 million scale)” by the U.S. National Institutes of Health (NIH) in 2022 and the massive investment in Altos Labs, a cell senescence-based therapeutic development company founded by Amazon founder Jeff Bezos. Although targeting senescent cells is gaining attention as a key to victory in the “war against human aging,” in order to commercialize the technology, an in-depth understanding of the characteristics of senescent cells and the identification of control methods are necessary.
The research team focused on the fact that senescent cells, despite being non-dividing, maintain high activity in nutrient-energy metabolic pathways. They newly discovered that this characteristic of senescent cells is regulated by cholesterol that specifically accumulates in lysosomes, known as the cell’s “degradation factories.” They confirmed that inhibiting this pathway can significantly suppress the production of harmful inflammatory factors secreted from senescent cells.
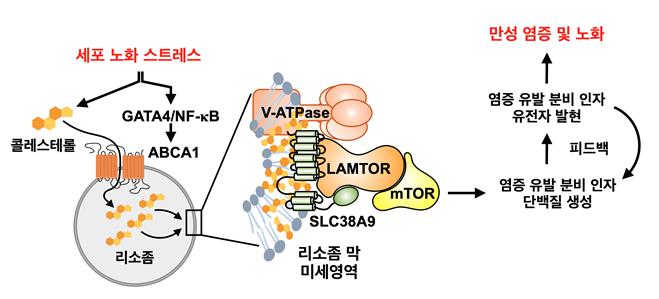 Various stresses that induce cellular senescence promote cholesterol metabolism within senescent cells while simultaneously changing the intracellular location of the cholesterol efflux factor ABCA1 from the cell membrane to the lysosome. ABCA1 located in the lysosome acts as a lysosomal cholesterol influx factor, accumulating cholesterol within the lysosome and forming lysosomal membrane microdomains. These lysosomal membrane microdomains enhance the activity of mTORC1, a key regulator of nutrient-energy metabolic pathways, and induce chronic inflammation and aging by promoting the production of pro-inflammatory secretory factors. Image provided by Seoul National University
Various stresses that induce cellular senescence promote cholesterol metabolism within senescent cells while simultaneously changing the intracellular location of the cholesterol efflux factor ABCA1 from the cell membrane to the lysosome. ABCA1 located in the lysosome acts as a lysosomal cholesterol influx factor, accumulating cholesterol within the lysosome and forming lysosomal membrane microdomains. These lysosomal membrane microdomains enhance the activity of mTORC1, a key regulator of nutrient-energy metabolic pathways, and induce chronic inflammation and aging by promoting the production of pro-inflammatory secretory factors. Image provided by Seoul National University
Using their independently developed “ultra-fast lysosome analysis technology,” the research team also identified ABCA1, a key factor in the mechanism of cholesterol accumulation in lysosomes of senescent cells. The accumulated cholesterol induces specific structural changes in lysosomes, promoting the interaction of nutrient-energy metabolic pathway regulators and causing abnormal activation. Furthermore, they verified that controlling the activity of ABCA1 to regulate the production of aging-related inflammatory factors can greatly contribute to alleviating symptoms of degenerative arthritis, a representative aging-related disease.
Professor Changhee Kang stated, “The significance of this research lies in elucidating the cholesterol-induced aging mechanism, which has long been shrouded in mystery, through the regulation of cellular senescence. We will strive to establish efficient senescent cell targeting strategies using this knowledge.” He added, “The lysosome-specific accumulation of cholesterol can also occur in cancer cells, where abnormal regulation of nutrient-energy metabolic pathways is well documented.”
Professor Jinhong Kim also said, “Inhibitors of lysosome-specific ABCA1 activity can be applied as effective treatments for degenerative arthritis caused by aging-related inflammatory responses.” He added, “Since these inhibitors target cellular senescence, the fundamental unit of aging, there is a high possibility that they can be developed as treatments for a broader range of aging-related diseases.”
This study was published in the international metabolism journal Nature Metabolism.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















