[Asia Economy Reporter Kim Eunha] A patient who used artificial tears from an Indian pharmaceutical company was infected with Pseudomonas aeruginosa, leading to death in the United States.
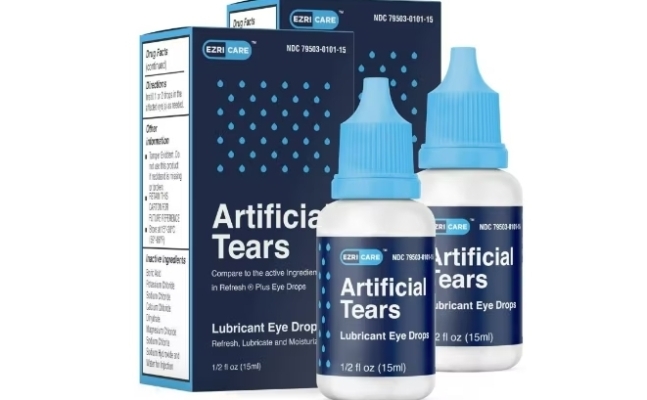
According to foreign media such as CNN and Reuters, Pseudomonas aeruginosa was detected in the artificial tear product ‘EzriCare’ from the pharmaceutical company Global Pharma, resulting in 55 people being infected with the bacteria, among whom 1 died and 5 lost their eyesight.
Accordingly, on the 1st (local time), the U.S. Centers for Disease Control and Prevention (CDC) recommended discontinuing use, and the U.S. Food and Drug Administration (FDA) issued an import alert on the product for failing to comply with CGMP (Current Good Manufacturing Practice) requirements. The pharmaceutical company voluntarily began recalling the product from the 2nd.
Pseudomonas aeruginosa can infect not only the eyes but also the lungs and bloodstream, and can reach the lungs and bloodstream through the nasal cavity connected to the eyes. The person who died after using this product actually died because the bacteria reached the bloodstream.
The product was available over the counter without a doctor's prescription and did not contain preservatives to prevent bacterial growth. U.S. health authorities recommend using prescription eye drops prescribed by a doctor rather than over-the-counter artificial tears.
The CDC advised that if greenish or clear discharge comes from the eyes or if there is eye pain after using the product, it may be infected and one should get tested. Blurred vision or eye redness are also side effects.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















