Collaboration with US Guardant Health
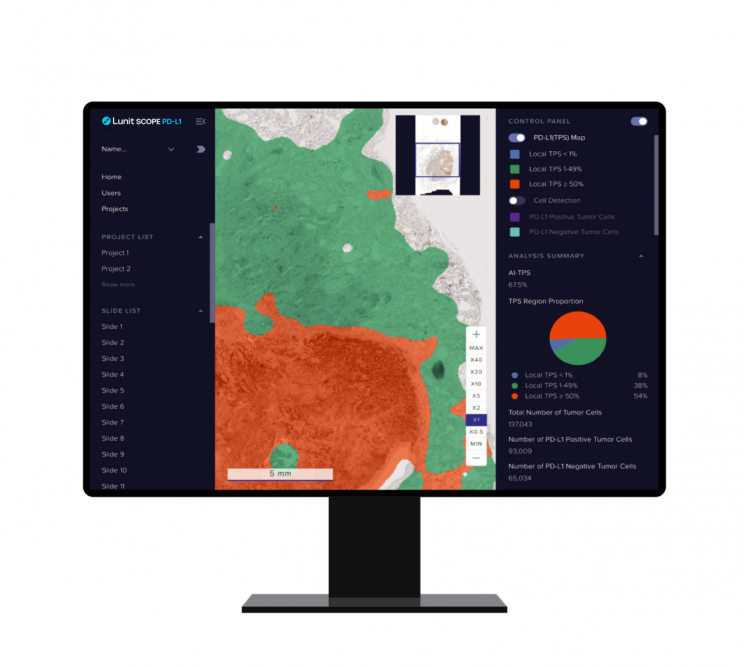
Utilizing AI Pathology Analysis Solution 'Lunit Scope PD-L1'
"Completing Product Portfolio from Cancer Diagnosis to Treatment"
[Asia Economy Reporter Myunghwan Lee] Lunit announced on the 1st that it has unveiled the AI-based pathology analysis solution 'Guardant360 TissueNext,' developed in collaboration with the U.S. biohealthcare company Guardant Health, to the global market. Lunit has officially launched its product for the first time in the cancer treatment field, beyond existing cancer diagnosis.
With this launch, Lunit completes a product portfolio covering the entire cycle from cancer diagnosis to treatment and simultaneously makes a full-scale entry into the global market, including the United States.
Guardant360 TissueNext is the first portfolio of Guardant Health's comprehensive cancer screening project 'Guardant Galaxy,' applying the AI technology of Lunit's AI-based pathology analysis solution 'Lunit Scope PD-L1.'
PD-L1 (Programmed death-ligand 1) is a protein on the surface of cancer cells, characterized by its ability to predict the therapeutic effect of immune checkpoint inhibitors based on its expression level. In particular, testing Guardant360 TissueNext on patients with non-small cell lung cancer (NSCLC) showed that the PD-L1 detection rate in the target patients improved by more than 20%. Lunit explained that this means Guardant360 TissueNext analyzes the degree of PD-L1 expression more accurately and objectively, identifying additional patients who may respond to immune checkpoint inhibitor therapy.
Since signing an exclusive partnership in 2021, Lunit and Guardant Health have developed the AI-based Guardant360 TissueNext as their first joint product. Given that more than 80% of U.S. hematology-oncology specialists use Guardant Health products, they plan to accelerate sales targeting the U.S. and global markets.
Helmi El Tukki, Co-CEO of Guardant Health, said, "Lunit Scope has already demonstrated excellent PD-L1 detection capability in non-small cell lung cancer tests, showing potential to improve treatment decisions for cancer patients. Guardant Health will continue to enhance cancer screening functions and provide more accurate and useful information to medical professionals through the Guardant Galaxy project."
Seobum Seok, CEO of Lunit, said, "Guardant360 TissueNext is expected to improve the accuracy and efficiency of cancer diagnosis and help specialists find the most suitable treatment for patients. Since Guardant Health has secured large-scale distribution and sales channels in the U.S., we will strengthen our partnership and actively target the U.S. and global markets."
Guardant Health, listed on the NASDAQ, is a biohealthcare company with a market capitalization of about 4 trillion KRW. After signing an exclusive partnership for Lunit's AI biomarker platform Lunit Scope, Guardant Health has recently expanded its business area into cancer tissue biopsy.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.