Moderna's 'mRNA-1345' Phase 3 Clinical Trial Success
FDA Approval Application Planned in First Half of Year
GSK and Pfizer to Decide FDA Approval in May
SK Bioscience and Ubio Leading Development in Korea
[Asia Economy Reporter Lee Chun-hee] Respiratory syncytial virus (RSV) is a representative seasonal respiratory disease that spreads globally every winter, raising concerns such as the "twin demic (flu)" and "triple demic (flu and COVID-19)." However, since no preventive vaccine has been developed yet, global pharmaceutical companies are entering the field, and the first vaccine launch is coming into sight.
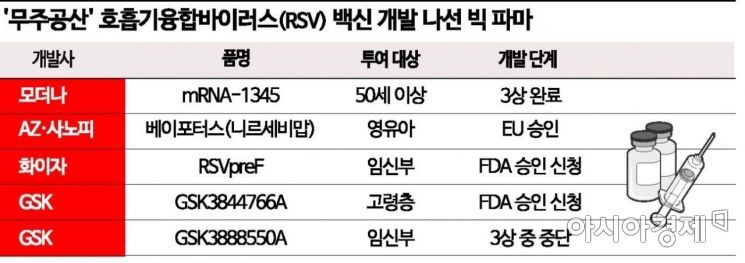
According to the industry on the 19th, Moderna, a company specializing in messenger ribonucleic acid (mRNA) therapeutics and vaccines, announced on the 17th (local time) that its RSV vaccine under development, 'mRNA-1345,' met the primary endpoint in a Phase 3 clinical trial targeting the elderly. In this clinical trial conducted on over 37,000 people aged 60 and above across 22 countries including the United States, mRNA-1345 demonstrated an efficacy of 83.7% in preventing lower respiratory tract diseases associated with RSV. Moderna CEO St?phane Bancel said, "The clinical data is encouraging," adding, "This is the second case where Moderna's mRNA pandemic vaccine platform has produced positive Phase 3 clinical results following the COVID-19 vaccine 'Spikevax.'"
Based on this clinical trial, Moderna plans to apply for FDA approval of mRNA-1345 within the first half of the year. mRNA-1345 was also designated as a 'Fast Track' by the FDA in 2021, so a swift review is expected. Additionally, Moderna is conducting clinical trials targeting those aged 50 and above to expand the indications for mRNA-1345, while also developing a trivalent vaccine 'mRNA-1230' that can prevent RSV, COVID-19, and influenza simultaneously.
RSV is a highly contagious seasonal respiratory virus and is considered a major cause of lower respiratory tract infections and pneumonia. It poses a significant health threat especially to infants and the elderly. As of 2019, approximately 5.2 million cases of RSV infection occurred among adults aged 60 and older in high-income countries, with about 500,000 hospitalizations reported. Major complications include respiratory distress, pneumonia, bronchitis, hospitalization, and death. Beyond acute infection, RSV can exacerbate underlying conditions such as asthma and chronic obstructive pulmonary disease, and may lead to acute myocardial infarction, stroke, and long-term respiratory function decline.
However, aside from the RSV preventive antibody treatment for infants and young children 'Beyfortus' (active ingredient nirsevimab), jointly developed by AstraZeneca (AZ) and Sanofi, which received approval from the European Medicines Agency (EMA) in September last year, there are no internationally approved preventive drugs. Notably, no FDA-approved drugs exist yet, intensifying the development competition among global pharmaceutical companies targeting this area.
GlaxoSmithKline (GSK) has applied for FDA approval of its vaccine for the elderly, 'RSVPreF3 (GSK3844766A).' Phase 3 clinical trials confirmed overall efficacy against RSV lower respiratory tract infections in adults aged 60 and above. Designated as a priority review by the FDA, the approval decision is expected in May. GSK has also applied for approval from the EMA and Japan's Ministry of Health, Labour and Welfare.
Pfizer completed Phase 3 clinical trials of 'RSVpreF (PF-06928316),' which is administered to pregnant women to confer immunity to the fetus, and has submitted it for FDA review. It showed an 81.8% prevention rate for severe lower respiratory tract infections and 57.1% prevention for all lower respiratory tract infections in infants within 90 days of birth. The FDA approval decision is also expected in May.
In South Korea, SK Bioscience is preparing to develop an RSV vaccine. Although still in the basic research stage, the company recently partnered with the Coalition for Epidemic Preparedness Innovations (CEPI) to develop an RSV vaccine using an mRNA vaccine platform. Additionally, UbioLogics and Ilyang Pharmaceutical are conducting RSV-related research.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.