Research Results of the Korea Research-based Pharmaceutical Industry Association (KRPIA)

[Asia Economy Reporter Myunghwan Lee] The average time required for the approval and review of new drugs in South Korea was found to be approximately 314 days.
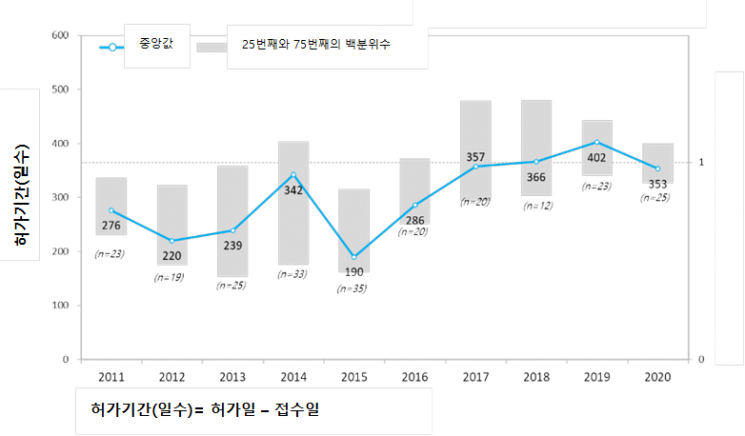
The Korea Research-based Pharmaceutical Industry Association (KRPIA) announced on the 16th that it conducted a "Study on the Approval Period of New Drugs in Korea" targeting 235 new drugs approved by global pharmaceutical companies in Korea over the past decade (2011?2020).
According to the results of this study, the average time taken for approval and review during the investigation period was 313.7 days, showing a steady increase since 2015. Compared to the average of 299.7 days from previous studies up to 2017, this represents an increase of about two weeks.
By drug type, synthetic drugs took 291.0 days, while biopharmaceuticals took 353.0 days. Regression analysis showed that biopharmaceuticals took on average 43.2 days longer than synthetic drugs.
In the comparative analysis by new drug efficacy groups, anticancer drugs accounted for the largest number of approvals with 92 cases (39.1%). Although differences in approval periods among efficacy groups were not significant, the approval and review period for anticancer drugs was somewhat faster.
Orphan drugs, which account for 37.4% of all new drugs, had 88 items approved by 2020. The approval period for orphan drugs was on average 130.4 days faster compared to general items.
However, despite the enactment of the "Rare Disease Management Act" in 2015 to promote the development of orphan drugs and enhance patient access, the approval and review period for orphan drugs increased after 2016. Orphan drugs require a separate designation process, which extended the total time needed for complaint review. This was explained as being due to multiple factors, including the implementation of Good Manufacturing Practice (GMP) inspections and risk management plans for orphan drugs effective from July 1, 2015, and the increase in biopharmaceuticals.
Through this study, the association explained that the GMP inspection period during the approval and review process was found to take longer on average than safety and efficacy reviews or standards and test method reviews. In particular, whether on-site GMP inspections were conducted and their scheduling had a significant impact on the approval review period.
Meanwhile, it was noted that despite the early COVID-19 pandemic situation in 2020, the approval review period actually decreased compared to the previous year. This was presumed to be mainly due to the exemption of on-site inspections at manufacturing sites during that period.
The association stated, "Although there have been various efforts to improve expedited approval and review systems such as priority review and conditional approval following the revision of the Pharmaceutical Affairs Act on July 20, 2021, continuous efforts are needed to shorten the approval period, including expanding specialized personnel in review departments, harmonizing related regulations internationally, strengthening cooperation with foreign regulatory agencies, and expanding mutual recognition systems."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.