Possessing Domestic Vaccines and Therapeutics
But Follow-up Development Stagnates
Endemic and Declining Profitability
Leading to Frequent Development Halts
 The first domestically produced COVID-19 treatment, Celltrion's 'Rekkirona' (left), and the vaccine 'Skycovione' by SK Bioscience (Photo by Celltrion, SK Bioscience)
The first domestically produced COVID-19 treatment, Celltrion's 'Rekkirona' (left), and the vaccine 'Skycovione' by SK Bioscience (Photo by Celltrion, SK Bioscience)
[Asia Economy Reporter Lee Chun-hee] Ildong Pharmaceutical's domestic emergency use authorization (EUA) for the oral COVID-19 treatment 'Jokoba,' developed jointly with Japan's Shionogi, has fallen through, delaying the emergence of a 'second' domestic COVID-19 treatment. Although follow-up development of vaccines and treatments continues, the transition to endemicity (periodic outbreaks of infectious diseases) has led to repeated clinical trial suspensions, causing difficulties.
According to the Ministry of Food and Drug Safety on the 29th, a total of 79 clinical trials related to COVID-19 vaccines and treatments have been approved domestically to date. Among these, excluding investigator-initiated trials or purely foreign-developed ones, only two have completed Phase 3 trials and development: Celltrion's treatment 'Rekkirona' and SK Bioscience's vaccine 'Skycovione.'
Although South Korea became the third country in the world, after the United States and the United Kingdom, to successfully develop vaccines and treatments domestically, news of a second vaccine or treatment development has not been heard. Moreover, the first products are facing challenges: production of Rekkirona has been halted, and Skycovione is struggling to enter the market. 'Jokoba (S-217622),' jointly developed by Ildong Pharmaceutical and Japan's Shionogi and already granted EUA in Japan, attempted commercialization based on Phase 2 and 3 clinical trial results conducted including in Korea, but for now, it has met with failure.
 Ildong Pharmaceutical's oral COVID-19 treatment 'Zocova,' jointly developed with Japan's Shionogi [Image source=Reuters Yonhap News]
Ildong Pharmaceutical's oral COVID-19 treatment 'Zocova,' jointly developed with Japan's Shionogi [Image source=Reuters Yonhap News]
Recently, the number of companies halting clinical trials continues to increase. Daewoong Pharmaceutical, Cellid, Celltrion, Chong Kun Dang, HK Innoen, CrystalGenomics, Samchundang Pharmaceutical, Genexine, Qurient, and Dong Wha Pharmaceutical have all declared discontinuation of COVID-19 related development this year. They cited difficulties in patient recruitment due to the endemic phase with decreasing COVID-19 cases and declining market potential as reasons.
On the 9th, Daewoong Pharmaceutical announced the suspension of its Phase 3 clinical trial in Korea for 'Camostat (DWJ1248),' developed as a COVID-19 treatment. Although three trials targeting mild to moderate patients, severe patients, and severe prevention were simultaneously conducted, they were sequentially halted, culminating in the final withdrawal of the trial targeting severe patients.
The obstacle was ultimately business viability. The company stated, "Due to the rapidly changing COVID-19 situation and expanded vaccination, the transition rate to severe patients is decreasing, making it difficult to secure clinical trial results," adding, "A change in development strategy is inevitable, and based on expert opinions and assessments of business viability relative to investment, this clinical trial is being discontinued."
The same applies to vaccines. On the same day, Cellid also announced the suspension of clinical trials for the COVID-19 vaccine 'AdCLD-CoV19-1.' They explained, "Due to the global spread of Omicron subvariants, domestic and international antibody prevalence and vaccination rates are increasing," and "there are difficulties in recruiting clinical trial participants, leading to the decision to terminate the trial early."
However, they emphasized that this does not mean a complete halt to related plans, stating, "Vaccine development targeting unvaccinated individuals is unlikely to be economically viable compared to booster vaccines," and "we intend to focus on developing the booster vaccine 'AdCLD-CoV19-1 OMI.'" This vaccine is being developed as a booster following Pfizer or Moderna vaccinations.
However, as of midnight the previous day, among 74,759 new third-dose recipients, 59,185 (79.2%) received the BA4.5-targeted vaccine, indicating that the BA4.5 vaccine has already become mainstream. This raises concerns that the business viability of the original Omicron variant vaccine may not be high.
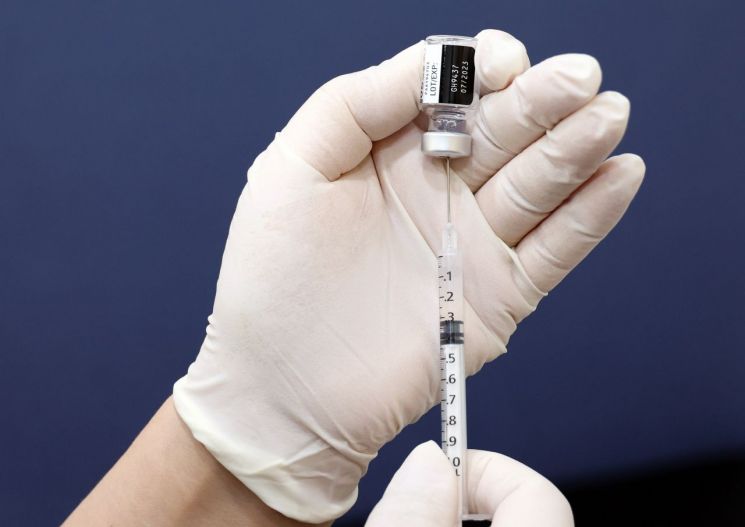 Medical staff at a hospital in Seoul are preparing for the additional COVID-19 winter vaccination.
Medical staff at a hospital in Seoul are preparing for the additional COVID-19 winter vaccination. [Image source=Yonhap News]
Nonetheless, some companies are still pursuing vaccine and treatment development. Shinpung Pharmaceutical (Piramex tablets), Genensel (ES16001), Immunemed (hzVSF-v13), and Shaperon (HY209) are conducting Phase 3 clinical trials for treatments. In vaccines, UbioLogics (Ucovac-19), Egen (EG-COVID), and GeneOne Life Science (GLS-5310) continue development. However, despite Shinpung Pharmaceutical receiving Phase 3 approval last August for 1,420 participants, it has yet to complete the trial, and these companies are also reportedly facing ongoing difficulties in patient recruitment due to endemic effects.
An industry insider said, "If patient recruitment is not completed by early next year, it will be practically impossible to continue ongoing vaccine and treatment development," expressing concern that "given the realistically lowered business viability, many companies may discontinue development next year as well."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















