Interview with Im Jin-hwan, CEO of AimMed
Ministry of Health and Welfare's 'Integrated Review of Innovative Medical Devices'
AimMed's 'Somz' Selected
"It Could Have Been Confusing, but Now We Can Focus on One Policy"
"Market Launch Expected in Q2... Development of Next Product Line Also Underway"
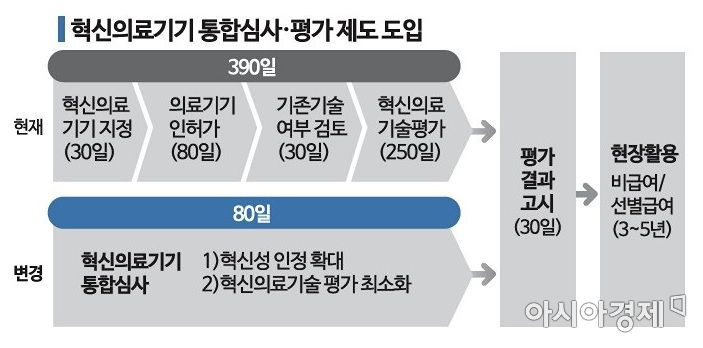
[Asia Economy Reporter Chunhee Lee] '390 days → 80 days'. Even with the release of the first digital therapeutic device (DTx) approved by the Ministry of Food and Drug Safety, a green light has been turned on for the DTx industry, which had an uncertain commercialization outlook. This is because the commercialization process, which used to take over a year, is expected to be drastically shortened to 80 days through the Ministry of Health and Welfare's introduction of the 'Integrated Review and Evaluation System for Innovative Medical Devices.'
On the 23rd, Lim Jinhwan, CEO of AimMed, said in an interview with Asia Economy, "Previously, we expected the market entry of 'Somz' to be as early as the first half of 2024," adding, "Through the integrated review system for innovative medical devices, we have received a great benefit by being able to advance this by about a year." Somz, a DTx for improving chronic insomnia developed by AimMed, was selected as an innovative medical device along with Welt's 'PillowRx' through this integrated review and evaluation system.
Recently, the Ministry of Health and Welfare unified the existing innovative technology medical device system?which required separate processes such as medical device approval by the Ministry of Food and Drug Safety (up to 80 days) and innovative medical technology evaluation by the Korea Health Industry Development Institute (up to 250 days)?into the integrated review and evaluation system for innovative medical devices. The prescribed period alone was over 390 days, more than a year, and if delays occurred in the middle, it could take several years until final release. However, going forward, once designated as an innovative medical device, commercialization will be possible within a significantly shortened timeframe.
CEO Lim emphasized, "Without the integrated review system, it would have been a confusing situation where one had to choose among several options such as new medical technology evaluation and innovative medical technology projects," adding, "With the introduction of the integrated review system, we can now focus on a single policy."
Currently, Somz is undergoing the medical device approval process by the Ministry of Food and Drug Safety, and once approved, it will go through the notification procedure that starts simultaneously, allowing market entry as early as 30 days after approval. It is known that considerable procedures have been completed, and there are prospects that approval could be granted within this year. Considering this, a launch in early next year is also anticipated.
CEO Lim expressed his ambition, saying, "If product approval within this year and approval of the innovative medical technology project are completed by the first quarter of next year, we plan to enter actual medical sites from the second quarter." Under the calculation that DTx which quickly accumulates real-world data (RWD) and analyzes feedback will have competitiveness, they are planning a rapid market entry.
He emphasized the importance of 'network' in the market entry process. CEO Lim said, "Collecting data is important, but smooth cooperation is crucial to actively induce participation from medical staff and patients," adding, "We will consult with major base hospitals including Seoul National University Hospital, Seoul Samsung Hospital, and Korea University Anam Hospital, where Somz clinical trials were conducted."
Finally, CEO Lim said, "We have already started the advancement of Somz and the development of the next product line," and added, "Please watch the chemical reaction that AimMed will create in the market."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.