MFDS, HIRA, NECA, and Others
'Innovative Medical Devices' Previously Required Multiple Reviews
Integrated Review System Completes Review in 80 Days
Market Entry Possible 30 Days After MFDS Approval
'First Innovative Medical Devices' JLK, AimMed, Welt
JLK "Will Enable More Innovative Technology Challenges"
AimMed "Can Advance Launch by Over a Year"
Welt "A Milestone for Technology Development... Welcomes Market-Friendly System Introduction"
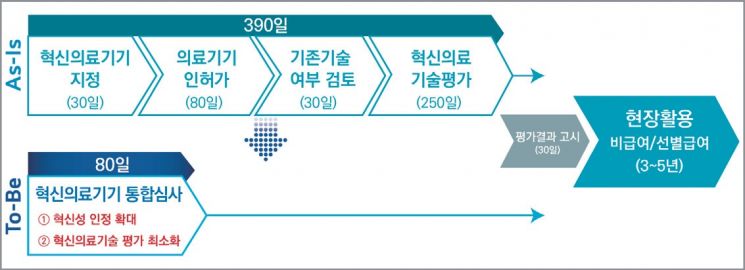 Integrated Review System Diagram for Innovative Medical Devices (Photo by Ministry of Food and Drug Safety)
Integrated Review System Diagram for Innovative Medical Devices (Photo by Ministry of Food and Drug Safety)
[Asia Economy Reporters Chunhee Lee and Seonjin Byun] One of the long-standing challenges in the non-invasive innovative medical device industry, including artificial intelligence (AI) diagnostic assistance and digital therapeutics (DTx), has been resolved. Previously, market entry took at least one year, but going forward, it is expected to be possible within as little as 80 days.
According to the industry on the 23rd, the Ministry of Health and Welfare recently introduced the 'Integrated Review and Evaluation System for Innovative Medical Devices' and designated the first target medical devices. The selected products are JLK's stroke diagnosis assistance software 'JBS-01K' and AimMed and Welt's chronic insomnia improvement DTx 'Somz' and 'Pillow Rx.'
Previously, medical devices applying 'innovative technology' had to undergo approval from the Ministry of Food and Drug Safety (up to 80 days) and innovative medical technology evaluation by the Korea Health Industry Development Institute (up to 250 days) for actual use. The prescribed period alone was about 390 days, exceeding one year, and if delays occurred in between, it was common for the final launch to take several years, causing widespread dissatisfaction.
In response, the Ministry of Health and Welfare integrated the related procedures into a single review and evaluation process, simplified evaluation criteria such as clinical usefulness and improvement of medical outcomes, and established this system to enable innovative medical devices to be rapidly introduced into medical settings. Products designated as innovative medical devices can be used in medical settings as non-reimbursed or selectively reimbursed for 3 to 5 years after undergoing a 30-day public notification process simultaneously with approval from the Ministry of Food and Drug Safety.
Representatives of the three companies designated as innovative medical devices all agreed that the system enables faster market entry. Kim Dong-min, CEO of JLK, expressed expectations that "an environment will be established where companies can challenge more innovative technologies." AimMed CEO Lim Jin-hwan said, "We can now focus on a single policy, moving away from a confusing situation," and Welt CEO Kang Sung-ji stated, "A market-friendly system has been introduced that allows rapid accumulation of real-world data (RWD) and development of devices."
JLK: "Usable from the end of next month... Enables challenges to more innovative technologies"
Kim Dong-min, CEO of JLK, said, "With the integrated review passed, non-reimbursed use of JBS-01K within hospitals will be possible from the end of next month," adding, "Accelerated market entry and enhanced business stability will create an environment where companies can challenge more innovative technologies."
JBS-01K is a medical device that analyzes patients' magnetic resonance imaging (MRI) scans and medical data using AI to classify the causes of stroke. Kim emphasized, "It helps classify stroke types and establish preventive treatments, enabling personalized treatment for patients."
Although JBS-01K was approved by the Ministry of Food and Drug Safety four years ago, commercialization was difficult due to uncertainty about insurance reimbursement. Kim said, "With this designation, it can be billed as non-reimbursed for 3 to 5 years, allowing use by many patients and enabling field verification," adding, "We will make various efforts to allow more people to use it."
AimMed: "Able to advance launch by one year... Will rapidly accumulate RWD"
Lim Jin-hwan, CEO of AimMed, said, "Previously, we expected Somz to enter the market in the first half of 2024 at the earliest," adding, "Now, we can advance this by about one year, which is a great benefit allowing Somz to be quickly prescribed and validated in actual medical settings."
He emphasized, "It was a confusing situation where we had to choose among several options such as new medical technology evaluation and innovative medical technology projects," adding, "With the introduction of the integrated review system, we can focus on a single policy."
Somz is currently undergoing product approval procedures with the Ministry of Food and Drug Safety. There is anticipation that approval may be granted within this year. Lim expressed his ambition, saying, "If product approval is obtained within this year and approval for the innovative medical technology project is completed in the first quarter of next year, we plan to enter actual medical settings from the second quarter." The plan is to enter the market quickly based on the calculation that DTx which accumulates RWD and analyzes feedback will have competitiveness.
Welt: "Welcomes market-friendly system introduction... Reimbursement should also be included"
Kang Sung-ji, CEO of Welt, said, "DTx is a software medical device (SaMD) that evolves based on RWD tailored to individual patients," evaluating, "It is significant that a market-friendly system has been introduced that simplifies verification to enable rapid launch, securing RWD and advancing device development."
However, he suggested, "Germany's 'Digital Health Application Program (DiGA)' also includes reimbursement aspects," adding, "Ultimately, since the most important thing is the pathway for use in the field, for the system to have more impact, reimbursement aspects should also be included in the system."
While undergoing review for Pillow Rx under this system, CEO Kang said, "There was some burden in completing processes that would have been done step-by-step all at once," but added, "I prepared diligently believing that graduating quickly would lead to better outcomes." However, he also emphasized, "Clinical environments and other factors do not seem mature enough for doctors and patients to smoothly utilize DTx," and stated that they will not rush commercialization.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.



![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)










