NASH New Drug That Big Pharma Struggled With
US Madrigal's 'Resmetirom' Succeeds in Phase 3 Trial
Meets All Primary Endpoints Set by FDA
Projected to Grow to 35 Trillion KRW by 2029
Fierce Development Competition in Korea
Hanmi, Yuhan, Dong-A, LG, Ildong Participate
[Asia Economy Reporter Lee Chun-hee] For the first time in history, a NASH (Non-Alcoholic Steatohepatitis) treatment has succeeded in clinical phase 3 by improving both fatty liver and fibrosis. As the number of patients continues to rise but no adequate treatment exists, this disease, once called the "graveyard of global big pharma," is now generating high market expectations.
According to industry sources on the 21st, Madrigal Pharmaceuticals in the U.S. announced on the previous day (local time) that its new drug candidate "Resmetirom" achieved top-line results meeting all primary efficacy endpoints in the NASH phase 3 clinical trial. Resmetirom is an orally administered THR-beta agonist. This clinical trial involved 955 patients diagnosed with NASH, who were administered Resmetirom 80 mg or 100 mg for 52 weeks, and the efficacy and safety were analyzed for up to 54 months comparing the treatment groups with a placebo group.
Resmetirom became the first ever to satisfy both primary efficacy endpoints set by the U.S. Food and Drug Administration (FDA) for NASH phase 3 trials: "resolution of fatty liver without worsening liver fibrosis" and "improvement of fibrosis without worsening NASH." In fatty liver resolution, the treatment groups showed 26% and 30% resolution rates, outperforming the placebo group's 10%. In fibrosis improvement, the rates were 24% and 26%, better than the placebo group's 14%. Statistical significance was confirmed for all results.
Regarding the secondary endpoint of LDL cholesterol reduction, the treatment groups showed decreases of 12% and 16%, respectively, compared to a 1% increase in the placebo group, confirming efficacy. Serious adverse effects were similar across all groups at about 12%.
Madrigal plans to apply for accelerated approval from the FDA in the first half of next year. Following these results, Madrigal's stock price soared by an astonishing 268% from the previous closing price, closing at $234.83 on the day.
NASH refers to a condition where symptoms similar to alcoholic fatty liver appear even in people who do not drink or drink very little alcohol. It is considered a causative disease for cirrhosis and liver cancer. The prevalence is high, ranging from 10-24% in the general population and 58-74% in obese individuals. As the number of patients continues to increase, data analysis and consulting firm GlobalData projected that the NASH treatment market, which was $1.444 billion (about 186.1 billion KRW) in 2019, will grow to $27.2 billion (about 35 trillion KRW) by 2029.
However, due to difficulties in clearly identifying the cause and the enormous R&D costs, even large pharmaceutical companies have failed to develop effective treatments, making it known as the "graveyard of big pharma." Gilead Sciences faced a setback in 2019 when its phase 3 trial of "Selonsertib" failed to meet statistical significance, and Novo Nordisk's "Ozempic," noted for diabetes and dementia treatment, failed to improve liver fibrosis.
However, with Madrigal's success in phase 3, demand for additional treatment development is expected to surge. In particular, Viking Therapeutics, which is developing a THR-beta mechanism treatment similar to Resmetirom, is scheduled to announce phase 2b results for "VK2809" next year, drawing significant attention.
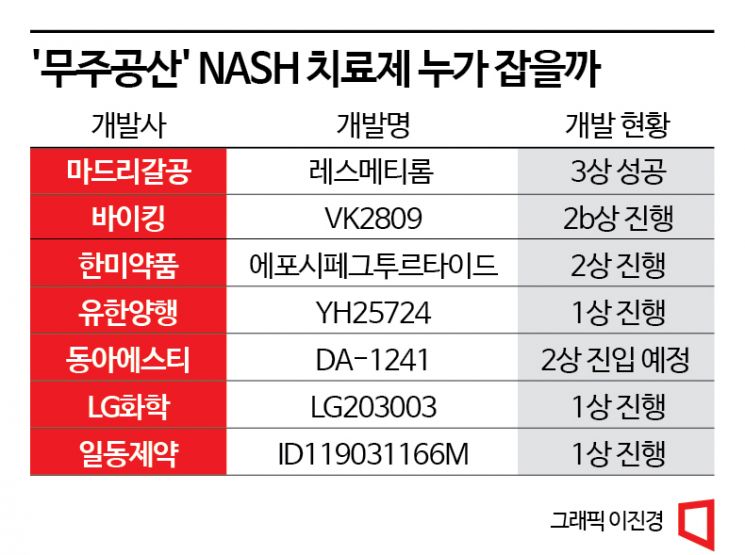
In South Korea, companies are also stepping up to the challenge. Hanmi Pharmaceutical, Yuhan Corporation, Dong-A ST, LG Chem, and Ildong Pharmaceutical are competing in development.
Hanmi Pharmaceutical has made NASH one of its core pipelines and is conducting global phase 2 clinical trials of "Eposipegtrutide (a triple agonist)" in the U.S. and Korea. This triple-action therapy targets glucagon, GIP, and GLP-1, and is being developed for various indications including NASH, primary biliary cholangitis (PBC), and idiopathic pulmonary fibrosis (IPF).
Yuhan Corporation is conducting phase 1 clinical trials of "YH25724," which it licensed out to Boehringer Ingelheim. This drug combines GLP-1 and FGF21 activities into a single molecule and is a long-acting protein drug developed using Genexine's sustained HyFc3 technology and Yuhan's proprietary protein engineering technology.
Dong-A ST acquired U.S.-based NeuroBo Pharmaceuticals and simultaneously licensed out "DA-1241," a NASH and type 2 diabetes treatment, and "DA-1726," a NASH and obesity treatment, to NeuroBo. DA-1241 has completed phase 1b trials in the U.S. and aims for global phase 2 next year, while DA-1726 has finished preclinical studies and plans to enter global phase 1.
LG Chem began dosing in a U.S. phase 1 trial in July for "LG203003," which selectively inhibits DGAT-2, an enzyme involved in triglyceride synthesis. Ildong Pharmaceutical received FDA approval for the phase 1 clinical trial plan (IND) for "ID119031166M" in July and is currently conducting the trial.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.