HK Innoen Confirms Formulation of 3rd Generation Smallpox Vaccine
Mico BioMed and SD Biosensor Obtain Export Approval
[Asia Economy Reporter Kim Young-won] As the number of confirmed monkeypox (Mpox) cases in South Korea rises to four, companies developing monkeypox vaccines and diagnostic products are accelerating their research, development, and exports.
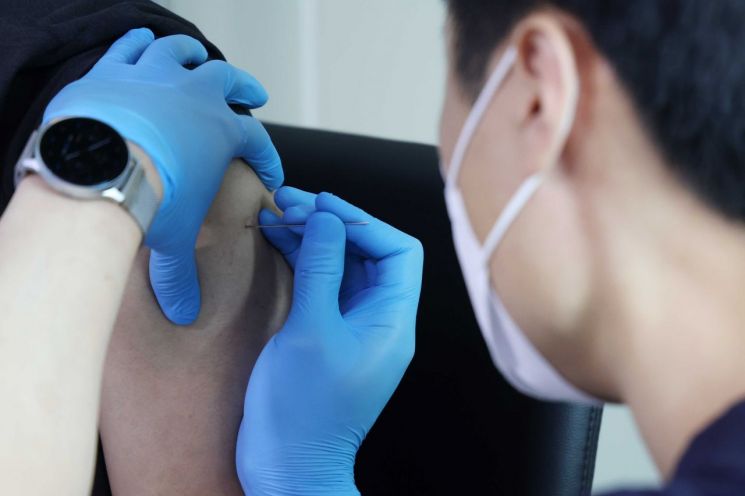
According to industry sources on the 30th, HK Innoen recently finalized the administration route of its third-generation smallpox vaccine effective against monkeypox as a subcutaneous (SC) injection formulation and is designing clinical trials for preclinical progress. HK Innoen has adopted a two-track strategy: expanding the use of its existing second-generation smallpox vaccine, approved in 2008, for monkeypox and developing the third-generation vaccine 'IN-B002.'
These vaccines were originally developed for human smallpox, not monkeypox. According to health authorities, the human smallpox vaccine provides about 85% preventive efficacy against monkeypox. Unlike the second-generation vaccine, which is administered by multiple skin punctures using a bifurcated needle, the third-generation vaccine can be injected like a regular shot. An HK Innoen official stated, "Expanding the indication for the second-generation vaccine requires future discussions with government agencies such as the Korea Disease Control and Prevention Agency, and preparations are underway to enter preclinical trials for the third-generation vaccine."
Currently, two companies have received export approval from the Ministry of Food and Drug Safety (MFDS) for monkeypox diagnostic products. Mikrobiomed obtained MFDS export approval in September for the country's first gene amplification (PCR) diagnostic kit capable of detecting monkeypox. Following this, SD Biosensor received export approval from MFDS for its monkeypox molecular diagnostic cartridge 'STANDARD M10 MPXV.'
Some companies have also started selling research-use-only (RUO) diagnostic products for research purposes before obtaining regulatory approval in various countries. Seegene developed the monkeypox diagnostic reagent 'Novaplex MPXV Assay' in June and is supplying it to overseas laboratories. A Seegene representative explained, "We are prioritizing and preparing regulatory approval applications for countries where actual human use is necessary." Bioneer also developed the monkeypox detection kit 'AccuPower Monkeypox Detection Kit' in June and is initially promoting its use as a research kit in overseas hospitals and laboratories.
Regarding monkeypox therapeutics, Hyundai Bioscience announced plans to apply for fast-track approval from the U.S. Food and Drug Administration (FDA) for 'CP-COV03,' an oral antiviral drug under development for COVID-19, as a monkeypox treatment. However, the company has not yet disclosed whether the application has been submitted.
According to the U.S. Centers for Disease Control and Prevention (CDC), as of the 28th, there have been 81,188 confirmed monkeypox cases worldwide this year, with about 15 new cases daily in the United States. On the 15th, the U.S. FDA granted Emergency Use Authorization (EUA) for Roche's monkeypox molecular diagnostic kit 'COBAS MPXV.' Earlier, the U.S. declared a public health emergency for monkeypox in August and issued EUA-related guidelines for monkeypox diagnostic products starting in September. To date, three products have received FDA EUA.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















