Japan Grants Emergency Use Authorization to Jokoba
Ildong Co-develops with Shionogi
Invested 108.2 Billion KRW in R&D Last Year
Yoon Woongseop: "19% of Sales Invested... R&D Will Be Central"
[Asia Economy Reporter Lee Chun-hee] The investments made by Ildong Pharmaceutical Group, which has focused on research and development (R&D), are gradually showing results. Following the approval of the COVID-19 treatment drug 'Zokova (S-217622)', co-developed with Japan's Shionogi Pharmaceutical, in Japan, the group is accelerating human clinical trials for various pipelines.
Ildong Pharmaceutical announced on the 23rd that Zokova received Emergency Use Authorization (EUA) from Japan's Ministry of Health, Labour and Welfare the day before. Zokova had previously applied for EUA in June and July but was put on hold. However, after the top-line results of Phase 3 clinical trials in September showed alleviation of COVID-19 symptoms in mild to moderate patients, it was approved on the third attempt.
Ildong Pharmaceutical is conducting domestic clinical trials for Zokova as part of the joint development. The company has secured exclusive domestic distribution rights and plans to promote domestic production after approval in Korea. Through this, they aim to create the first domestically produced oral COVID-19 treatment.
This approval is seen as the beginning of tangible results from Ildong Pharmaceutical's R&D investments, which have been actively pursued as the group's future growth engine since its transition to a holding company system in 2016. In 2017, Ildong Pharmaceutical also achieved approval for 'Vesibo', a chronic hepatitis B treatment, as the 28th domestically developed new drug.
Ildong Pharmaceutical's aggressive investment is also reflected in the numbers. Its R&D expenses increased from 48.3 billion KRW in 2017 to 108.2 billion KRW last year, more than doubling in four years. This year, it has already invested 93.8 billion KRW in just the first three quarters. Although operating profits have consecutively recorded losses due to increased R&D spending, the company continues bold investments.
Yoon Woong-seop, Vice Chairman of Ildong Pharmaceutical, stated at last month's World Bio Summit, "We aim to become an R&D-centered pharmaceutical company," adding, "Despite the significant impact of COVID-19 on the global economy, we invested 19% of last year's sales in R&D."
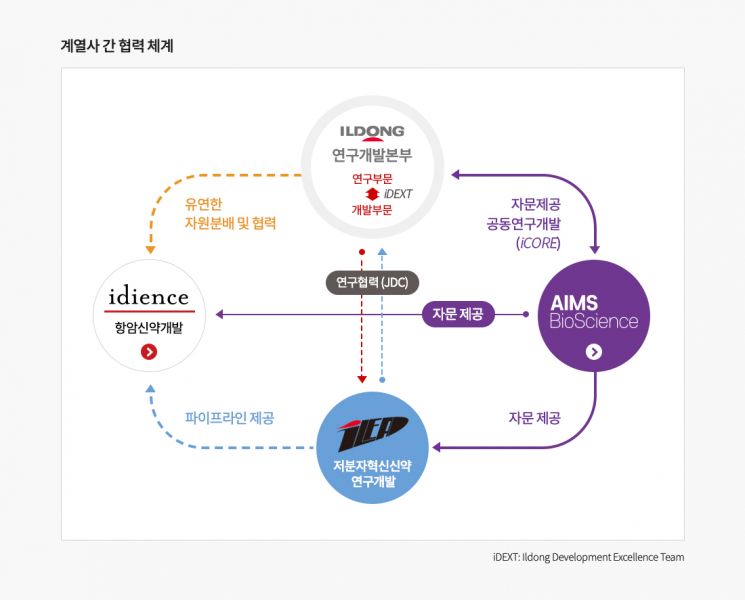
Another notable point is the establishment of an R&D ecosystem within the group. Ildong Pharmaceutical has subsidiaries such as Idience (oncology drug development), I'm Read (small molecule drug R&D), and Aims Bioscience (clinical pharmacology consulting), enabling all R&D processes to be conducted within the group. Recently, they decided to issue convertible bonds worth 30 billion KRW to provide financial support to Idience and Aims Bioscience.
As drugs are consecutively entering actual clinical stages, results are becoming visible. The most advanced is Idience's targeted anticancer drug 'Venadaparib (IDX-1197)', which has received Orphan Drug Designation (ODD) from the U.S. Food and Drug Administration (FDA) and is undergoing Phase 2 clinical trials.
Ildong Pharmaceutical itself is conducting Phase 1 clinical trials for the diabetes treatment 'IDG16177' currently in Germany. Additionally, the NASH treatment 'ID119031166' is in the U.S., and the P-CAB formulation 'ID120040002' for gastroesophageal reflux disease has received Phase 1 clinical trial approval (IND) in Korea.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.