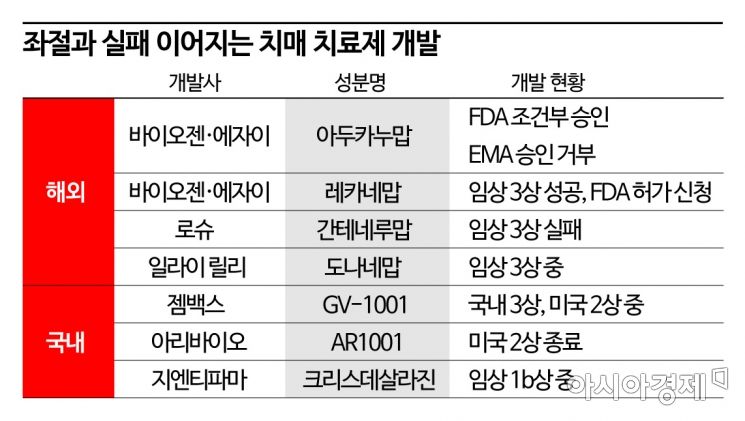
Roche's 'Gantenerumab' Phase 3 Failure
6-8% Improvement Confirmed... Statistical Proof Not Achieved
15-Year Project Possibly Doomed
Biogen & Eisai's 'Lecanemab' Phase 3 Success
FDA Approval Decision Expected January Next Year
Lilly's 'Donanemab' Phase 3 Ongoing
GemVax, Aribio, GNT Pharma Also Challenging Domestically
[Asia Economy Reporter Chunhee Lee] The dream of conquering dementia, which seemed to be succeeding, has once again faced setbacks. After the failure of Biogen and Eisai's Aduhelm (generic name aducanumab), the success of lecanemab's clinical trials seemed to give new momentum to dementia drug development, but Roche's gantenerumab has once again failed in Phase 3 trials.
On the 14th (local time), Roche announced that the top-line interim analysis of the Phase 3 'GRADUATE' study of gantenerumab in early Alzheimer's dementia patients failed to meet the primary efficacy endpoint. Gantenerumab is an amyloid-beta (Aβ) targeting antibody treatment for Alzheimer's dementia being developed by Roche and its subsidiary Genentech, based on technology licensed from German company Morphosys. Unlike other intravenous (IV) treatments such as Aduhelm, lecanemab, and Eli Lilly's donanemab, gantenerumab is administered via subcutaneous (SC) injection, which raised expectations due to its improved dosing convenience.
However, Roche announced that the Phase 3 trials, conducted under the names 'GRADUATE 1' (982 participants) and 'GRADUATE 2' (1016 participants), failed to achieve statistical significance for the primary endpoint. The primary endpoint was the change from baseline in the Clinical Dementia Rating-Sum of Boxes (CDR-SOB) after approximately two years (116 weeks).
The results showed an additional 6-8% improvement in CDR-SOB compared to the placebo group. However, the 'P-value' (statistical significance) to confirm that this was due to the actual efficacy of gantenerumab rather than chance was not met.
Thus, the clinical development of gantenerumab, which Roche has led for about 15 years, has faced another setback. In 2014, a Phase 3 trial targeting mild to moderate Alzheimer's patients also failed. Roche then attempted to re-challenge by changing the indication to mild cognitive impairment (MCI) or mild Alzheimer's patients, but again faced failure.
However, Roche is continuing various additional developments related to gantenerumab, so a complete halt in development is unlikely. Besides the 'GRADUATE' trial, Roche is conducting the 'SKYLINE' Phase 3 prevention trial involving 1,200 participants who show signs of Aβ accumulation but have not yet experienced cognitive decline. Additionally, a Phase 1 trial is underway for 'RG6102,' a bispecific antibody linked to a brain shuttle to enhance blood-brain barrier (BBB) penetration.
 Eisai (from the left) and Biogen logos, co-developers of 'Aduhelm' followed by 'Lecanemab' (Photo by each company)
Eisai (from the left) and Biogen logos, co-developers of 'Aduhelm' followed by 'Lecanemab' (Photo by each company)
With the failure of gantenerumab, the hopes for dementia treatment were somewhat dampened despite Biogen and Eisai's Aduhelm failing to enter the market, as lecanemab had demonstrated efficacy in Phase 3 trials. In September, Biogen and Eisai announced success in achieving the primary efficacy endpoint in the global Phase 3 trial of lecanemab, which they are co-developing. The endpoint was set as the change from baseline in CDR-SOB after 18 months of treatment, showing a 27% improvement compared to placebo. Statistical significance was also confirmed.
This has led to contrasting fortunes for the two parties. Both study results are scheduled to be presented at the Alzheimer's Clinical Trials (CTAD) International Conference in San Francisco from September 29 to October 2, highlighting the stark contrast. Lecanemab is also gaining momentum toward approval. In July, the U.S. Food and Drug Administration (FDA) accepted the biologics license application (BLA), with a decision expected by January 6 next year. Biogen and Eisai also plan to seek approval in Europe and Japan.
With the Phase 3 results of these two drugs released consecutively, the market is now focusing on Eli Lilly's donanemab as the next candidate. Currently in Phase 3 trials, it is expected to complete by the first half of next year. In Phase 2, donanemab showed a 32% improvement over placebo on the integrated Alzheimer's Disease Rating Scale (iADRS).
In South Korea, attempts to develop treatments continue. Companies such as GemVax, Aribio, and GNT Pharma are involved, mostly pursuing mechanisms targeting neuroinflammation removal or complex mechanisms rather than Aβ or tau. GemVax is conducting a domestic Phase 3 and a global Phase 2 trial of 'GV1001.' The Phase 2 trial is planned to be conducted simultaneously in the U.S. and seven European countries (Spain, France, Italy, Portugal, the Netherlands, Poland, Finland), with investigational new drug (IND) approvals already obtained in the U.S. and Spain.
Aribio, developing the oral drug 'AR1001,' has submitted an IND to the FDA to enter global Phase 3 trials. GNT Pharma, developing 'Crisdesalazine,' which applies the active ingredient commercialized as 'Zedacure' for canine cognitive dysfunction syndrome (CDS) to humans, has completed Phase 1 trials.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.