Only 13 Items Including Antipyretic Analgesics and Digestives Sold
Loophole Allows Unlimited Purchases at Multiple Locations
Convenience Store Industry Calls for Improved Access to Medicines
Pharmacists Suggest Expanding Public Late-Night Pharmacies as a Solution
 A customer is purchasing over-the-counter safety medicines at the convenience store CU. (Photo by BGF Retail)
A customer is purchasing over-the-counter safety medicines at the convenience store CU. (Photo by BGF Retail)
[Asia Economy Reporter Lim Chun-han] Since the COVID-19 pandemic, purchasing over-the-counter (OTC) drugs at convenience stores has become a daily routine, bringing the expansion of product categories into focus. While the convenience store industry argues that this is a necessary measure to enhance consumer convenience, the pharmaceutical community opposes it, citing concerns over misuse. Given that hundreds of adverse reaction reports related to OTC drugs are recorded annually, experts emphasize the need for a cautious approach.
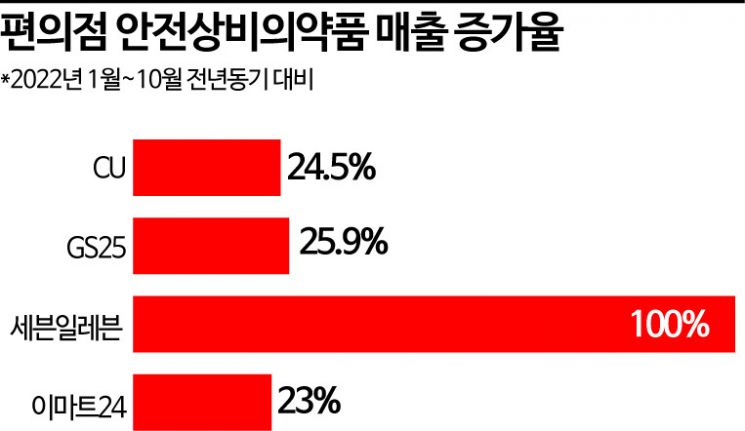
According to CU on the 8th, sales of OTC drugs increased by 24.5% from January to October this year compared to the same period last year. During the same period, GS25 saw a 25.9% increase, Seven-Eleven 100%, and Emart24 23%. Sales of OTC drugs have been steadily rising every year. At GS25, OTC drug sales grew by 23.4% in 2019, 29.3% in 2020, and 38.8% in 2021 compared to the previous years. Seven-Eleven also experienced growth of 10% in 2019, 10% in 2020, and 20% in 2021. Since the COVID-19 outbreak, the number of people purchasing medicine at convenience stores has surged, and demand for OTC drugs is expected to continue increasing.
Currently, there are 13 OTC drug items, including five types of antipyretic analgesics, two types of cold medicines, four types of digestive aids, and two types of patches. These are general medicines primarily used urgently for mild symptoms and can be self-administered by patients. However, these products differ from those sold at pharmacies in terms of product names and packaging units.
For example, Tylenol 500mg tablets are sold in packs of 10 at pharmacies but limited to 8 tablets at convenience stores. Since the maximum daily dose of acetaminophen is 4000mg, only a day's supply is sold to prevent misuse. There are also differences in the drug's ingredients and dosages, which are measures to minimize side effects. However, a loophole exists where consumers can purchase unlimited amounts by visiting multiple convenience stores.
The National Convenience Store Franchise Association argues that expanding the range of OTC drugs to include items such as antidiarrheals, antacids, and burn ointments is necessary to eliminate blind spots in public safety. Park Byung-wook, representative of the Emart24 Store Owners Association, emphasized at the 'Policy Improvement Seminar for Sustainable Development of Community-based Retail' held at the National Assembly in August, "During the COVID-19 situation, convenience stores played a public role by distributing antipyretics, masks, and self-test kits," adding, "In small provincial cities, convenience stores can improve access to medicines." According to a 2020 consumer monitoring survey by the Pharmaceutical Policy Research Institute, 56% of respondents agreed that the range of products sold at convenience stores should be expanded, with the highest demand for antidiarrheals, antacids, allergy medicines, and laxatives.
On the other hand, the pharmaceutical community counters that selling medicines at convenience stores can lead to drug misuse and side effects. A representative from the Korean Pharmaceutical Association stated, "Medicines are different from general consumer goods. Convenience alone is not the issue," adding, "Because misuse and side effects can occur, expert judgment is necessary rather than that of the general public. Issues with purchasing medicines during late-night hours should be addressed by expanding the operation of public late-night pharmacies."
Due to the stark differences in positions between the convenience store industry and the pharmaceutical community, discussions on expanding OTC drug categories have not progressed for four years. Since the sixth meeting in August 2018, the OTC Drug Designation Review Committee has effectively ceased activities. Under current law, OTC drugs are designated within a limit of 20 items, but no readjustment has been made since the designation of 13 items.
Professor Lee Young-ae of the Department of Consumer Studies at Incheon National University said, "It is true that convenience stores have better accessibility than pharmacies. If the medicines have no major side effects, selling them at convenience stores is beneficial from a consumer convenience perspective," but she also advised, "Unlike pharmacies, convenience stores cannot provide consultations about medicines, and misuse issues may arise, threatening consumer safety. The types and dosages of medicines sold should be determined through thorough discussions."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.