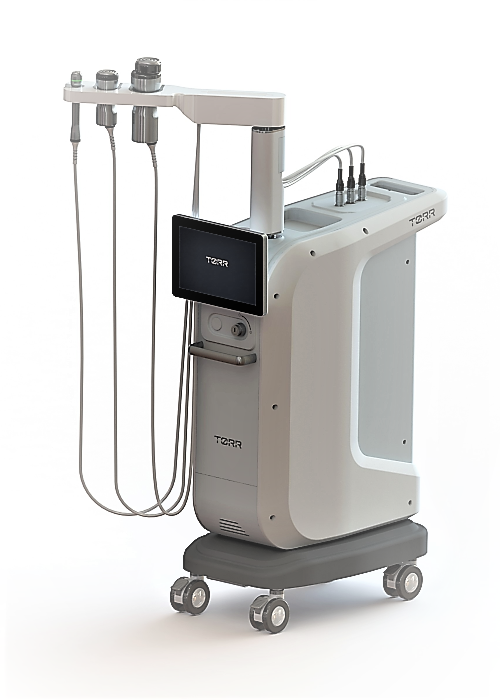
[Asia Economy Reporter Chunhee Lee] Iruda, a specialized company in beauty medical devices, announced on the 28th that its non-invasive radiofrequency (RF) device 'ToRR RF (model MTX-C1)' has been approved by the U.S. Food and Drug Administration (FDA). This marks the fourth FDA approval this year following Acutron, Secret Duo, and Lipot.
ToRR RF is a non-invasive radiofrequency device operating at a frequency band of 1 MHz. It can be applied diversely to treatment areas and indications through three different handpieces. Especially as it is a treatment device utilizing deep heat where temperature control is crucial, temperature sensors have been applied to the handpieces to significantly reduce the risk of side effects such as burns.
Existing RF devices applied energy by broadly rubbing the treatment area. In contrast, ToRR RF effectively penetrates energy through vibration and motion heads, generating deep heat quickly and effectively. In particular, the 'Deep/Low Mode,' which allows changing the electrode arrangement, enables varying penetration depths, making it applicable to various indications.
An Iruda official stated, “Starting with Acutron in 2022, followed by Lipot and ToRR RF, we have recently received FDA approvals for devices with various mechanisms, strengthening our brand recognition in the U.S., the largest market for skin beauty medical devices, and enabling us to establish a lineup of diverse products to target the market. Moving forward, we will achieve sales growth by strengthening sales globally, including in our main markets such as the U.S., Europe, the Middle East, and Asia.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.